Our Facebook Page to Follow: Aquarium/Pond Answers Facebook
This is a unique resource for answers, help, & advice to aquarium and pond questions not found elsewhere; With regular posts & article updates.
In our research; we use aquaculture, horticulture, medical, & university research to compile many of our articles.Our Recommended Lighting for highest efficiency professional planted/reef aquariums: "AquaRay Lighting"
Tap water in Aquarium/Pond; Chlorine/Chloramines, TDS, Vitamin C
By Carl Strohmeyer
Updated 1/22/19
Index (click to "Jump To")
- Chlorine & Chlorimines
- Vitamin C for Chlorine & Chlorimines
- Inorganic Molecules; Nitrites, Nitrates, Copper, Phosphates, and Fluoride
- TDS (Total Dissolved Solids)
- Sodium
- Summary & References
There are standards for tap water quality, but that does not mean that these levels are safe for fish (or humans for that matter).
CHLORINE AND CHLORAMINES:
To start, most city tap water has chlorine (Chlorine (Cl2), Sodium Hypochlorite NaClO), which is an oxidizer (A chemical substance that gains electrons in a redox chemical reaction), but this can kill fish by burning their gills and poisoning their blood.
More about: Aquarium Redox Balance
Chlorine is not very stable and is easily removed with the many commercial "De-chlorinators" available, most using Sodium Thiosulfate.
Agitation of the tap water in a bucket or other container with an air stone connected to an air pump will generally remove the amount of chlorine generally added to tap water in a matter of hours if not at least a day. However, if chloramines are used, agitation will not work for removal.
Some municipalities use chloramines because they are more stable than chlorine (this is especially common in areas where water must be transported over longer distances due to non-availability of local water sources, such as in the Southwest USA or areas of drought).
Chloramines (NH2Cl) are a chemical compound of chlorine and ammonia and cannot be boiled out. It can't even be allowed to sit for a few days to remove the Chloramines before adding this water to an aquarium.
Chloramine is formed through the reaction of dissolved chlorine gas and ammonia in tap water. Chloramines can also be composed of two other formulas: dichloramine (NHCl2) and trichloramine (NCl3).
Chloramine passes through the gills of fish and enters the blood stream. There, it reacts with Hemoglobin, forming Methemoglobin.
In studies of some fish exposed to 1 ppm-Cl of monochloramine, then about 30% of the hemoglobin is converted into methemoglobin; the fish suffered from anoxia (low oxygen in their tissues) because they have lost some of their hemoglobin, which is responsible for carrying oxygen in the blood.
In my experience, fish exposed to chloramine suffer immediate and often severe reactions from darting, to gasping, to immediate shock and death!
This is NOT the general reaction of exposure to chlorine, as fish generally do not show symptom of exposure to chlorine in normal tap water doses unless exposure is prolonged, and most de-chlorinators remove chlorine instantly/within seconds.
See a simple experiment in this article: “Aquarium (& Pond) Water Conditioners” (about three paragraphs down).
ADVERTISEMENT
 If your tap water has Cloramines, you will need to remove them chemically before adding the water to your aquarium. Standard de-chlorinators such as "Start Right" Water Conditioner will remove the chlorine, but leave the ammonia (NH3) for either your bio filtration or Zeolite (freshwater only) to remove. These basic de-chlorinating products are simple Reducers (sodium thiosulfate) and are quite safe, even overdosed contrary to some opinions floating around.
If your tap water has Cloramines, you will need to remove them chemically before adding the water to your aquarium. Standard de-chlorinators such as "Start Right" Water Conditioner will remove the chlorine, but leave the ammonia (NH3) for either your bio filtration or Zeolite (freshwater only) to remove. These basic de-chlorinating products are simple Reducers (sodium thiosulfate) and are quite safe, even overdosed contrary to some opinions floating around.
The vastly preferred products for use in conditioning water treated with Chloramines such as Amquel (or better Amquel Plus) or SeaChem Prime will remove the chlorine and neutralize the ammonia (and more).
Prime is made from Hydrosulfite salts which are basically non toxic reducing agents made up of bisulfites and hydrosulfites, aqueous solution, buffered at pH 8. As mentioned earlier, reducing agents are basically non toxic at reasonable doses to fish and aquatic animals.
A product resource for: SeaChem Prime
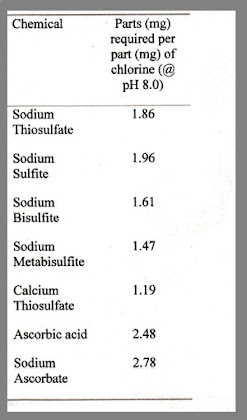
The chart to the right shows some common chlorine/chloramines reducing agents.
You will note that metabisulfites and bisulfites are efficient reducers, however it should be noted that some studies have shown these to lower dissolved oxygen levels. I have never had a problem with this due to the fact I always employ good circulation when ammonia, chloramines, or chlorine are a problem (actually good circulation should always be employed).
Also note, Vitamin C (ascorbic acid/sodium ascorbate) is also an reasonable reducer (albeit not as strong as others at doses that do not affect pH dramatically), which also goes along with many of my points for a Reducing Redox.
In fact, Vitamin C in either Ascorbic Acid or Sodium Ascorbate can effectively lower chlorine or break the chloramine bond. Generally, approximately 2.5 to 2.8 parts of Vitamin C is required to neutralize 1 part chlorine
Be aware that Vitamin C, in particular in the form of ascorbic acid, can dramatically lower pH in normal doses to be effective.
Vitamin C may be a good choice for 25% water changes where its lower reduction abilities at safer pH doses might even be desired.
HOWEVER, in larger water changes or especially with chloramines, the undesirable drop in pH at doses required may make it a poor or even dangerous choice. Ascorbic Acid at doses that may be required will immediately reduce pH by 1.5 on the logarithmic pH scale (which can shock or even kill fish when this change happens suddenly, which it will without adequate alkalinity via bicarbonate buffers). Sodium Ascorbate will change pH at doses required will immediately reduce pH by .5 (reference 1).
Nor is Vitamin C as a good a choice for long term Redox balance and reduction since it is a short term reducer.
For much more information about water conditioners that will remove Chlorine and/or Chloramines, please read this article:
Aquarium (& Pond) Water Conditioners.
Further Aquarium Redox Information:
Aquarium Redox
Other Methods for Chloramine Removal Include:
- Reverse Osmosis or Deionization Resins: reverse osmosis systems (where carbon is usually part of the pre-filtration prior to the RO membrane), the ammonia is partially removed by the reverse osmosis system. The extent of removal by the RO membrane depends on pH. At pH 7.5 or lower, reverse osmosis will remove ammonia from 1.4 ppm-Cl monochloramine to less than 0.1 ppm ammonia.
DI resin can then remove any residual ammonia.
This method is EXCELLENT for preparation of freshwater prior to adding a marine salt mix or for topping off aquariums prior to evaporation.
HOWEVER, it's not a good method for 100% use of water in freshwater aquariums as ALL important minerals are stripped from the water that freshwater fish need for osmoregulation (in marine tanks, the saltwater mixes provide these essential elements).
PLEASE read these articles fully before using this method for freshwater aquariums:
*AQUARIUM CHEMISTRY; Use of RO water in Freshwater Aquariums
* Use of RO, DI, Softwater in Aquariums
*PROPER OSMOTIC FUNCTION- ELECTROLYTES; Osmoregulation in Fish - Carbon & Zeolite: as with thiosulfate (used in may water conditioners such as Start Right, Novaqua, Tap Water Conditioner, etc.), carbon removes the chlorine, however ammonia is not bound significantly by activated carbon.
Consequently, treatment of water with activated carbon will need to be followed up by some method of eliminating the ammonia such as the use of Zeolite. Zeolite is NOT for use in saltwater, however it can be an effective albeit SLOW method of removing residual ammonia from the previous Chloramines molecules.
A Product Resource for: Ammo-Carb, Ammo-Chips, Zeolite Products - Even the use of a Category A or B True UV Sterilizer will slowly lower chlorine in an aquarium as per my tests, albeit not as fast as traditional water conditioners or Vitamin C.
Reference: Aquarium/Pond UV Sterilizer Use
INORGANIC CHEMICALS; Nitrites, Nitrates, Copper, Phosphates, and Fluoride:
Ammonia: As of the time of this article, there are no limits as per the amount of ammonia (either NH3 or NH4) allowed in tap water.
Reference: Ammonia in Drinking Water
Nitrites are allowed up to 1 ppm, yet at this level there can be some damage to fish gills. Methylene Blue (for nitrite and ammonia poisoning) can be used for treatment of nitrite poisoning, but it is best to avoid this. A good bio filter will generally remove trace amounts of this from tap water, as will products such as Prime.
Nitrates are allowed up to 10 ppm, yet at levels above 10- 30 (depending on studies) in human studies infants under 6 months can become ill and suffer symptoms such as Blue Baby Syndrome.
More about:
Aquarium Nitrates
Also See these links for more about Nitrates:
*www.thirteen.org/edonline/studentstake/water/schoolwater/nitrogen/nitrate.htm
Now this level has shown no ill effect in any fish studies I have seen, but levels above 20 ppm can harm some marine cephalopods.
It makes since in many marine aquariums too use RO water to mix up your salt mix or top off for evaporation so as to not add to "difficult to remove nitrates" in you marine aquarium.
Other "allowed chemicals" of note are Copper- 1.3 ppm, Phosphates (no standards) and Fluoride- 4.0 ppm.
Copper at these levels is not generally a problem with fish or aquatic invertebrates, but if you are already treating with copper sulfate or if this is allowed to accumulate in a reef tank this is something an aquarist should be aware of.
Copper levels above 5 ppm can start to become dangerous for some delicate invertebrates such as Acropora corals and levels above 25 ppm can be dangerous to fish. It also should be noted for copper, that in hot water in particular, copper can be also added to tap water via home copper plumbing.
As for Fluoride; I have not found conclusive studies on the harm of Fluoride to fish or other aquatic creatures, in fact trace amounts are necessary for coral growth in marine aquariums. So despite some over stated worries about Fluoride in tap water used in aquariums, this in one I would not consider.
As for Phosphates; many municipalities use phosphates to reduce the levels of lead that have been found in drinking water.
Phosphates create a protective film on the inside of the pipe, slowing the electrochemical processes that lead to corrosion.
Unfortunately for aquarists this can lead to extra algae growth, especially of Blue Green Algae (Cyanobacteria). This can be a real problem in both freshwater and saltwater aquariums without easy solutions.
I have used many phosphate sponges with mixed results, but I can say with certainty is that carbon will not remove phosphate, in fact some carbon may even add to your phosphate levels. Protein Skimmers in marine aquariums can remove some phosphates, but I have not recorded that much difference.
Water changes using RO water and then adding minor elements and electrolytes back in is another solution. In freshwater aquariums, Wonder Shells can help add these elements as well, but in saltwater the marine mixes have all the elements you need.
A resource for:
*“Wonder Shells – calcium and electrolyte replenisher”
*Rena Phos-Zorb
*NPX Bioplastics Nitrate & Phosphate Reducing Polymer Media
TDS (Total Dissolved Solids)
Total Dissolved Solids basically is any minerals, salts, metals, cations (positively charged mineral ions) or anions (negatively charged mineral ions) dissolved in water.
This includes anything present in water other than the pure water (H20) molecule and suspended solids. (Suspended solids are any particles/substances that are neither dissolved nor settled in the water, such as detritus).
The TDS is equal to the sum total of cations and anions ions in the water. Generally the measurement of TDS is given in Parts per Million (ppm) being the weight-to-weight ratio of any ion to water.
There of coarse is a relationship to GH (General Hardness) and Redox, so too low or too high a TDS can be detrimental, depending upon the fish kept. With this in mind, the use of RO (Reverse Osmosis Systems which lower TDS considerably should take into consideration the re-mineralization of the water (again depending upon the fish kept).
Also Redox is affected when one uses RO (or DI) water, as the Redox of RO Water is generally too high (since RO water is more acid oxygen is left behind), as a highly oxidizing environment may be OK for short term, long term health considerations for fish (or even humans as per Redox Research) is not good.
For Further in depth information about Redox:
Aquarium Redox
RO Machine Links:
Reverse Osmosis Water Filter
Aquarium Minerals for RO Water
The diagram below shows the relationship of TDS and Tap Water (from www.tdsmeter.com/)

Please Click on the Diagram Above to Enlarge
SODIUM:
The Environmental Protection Agency suggests 20 mg or less of sodium per liter as the amount of sodium to strive for in drinking water, so anything over this amount is going to tend toward driving out certain minerals, albeit in small amounts, that are essential for fish, such as Calcium & Magnesium. As freshwater sodium level increases beyond this number, it will become increasing difficult to maintain a healthy/balanced GH/KH.
A sodium level that is over 270 mg/liter in freshwater is considered quite high and at this level maintaining the correct essential mineral ions will be very difficult in your aquarium.
It is for this reason a sodium softened water system should NEVER be used for ANY aquarium.
See: Softened Water; Home/Office Water Softeners Use
SUMMARY:
Before you go and rush out and use nothing but bottled water, please note that most bottled water is NOT suitable for fish when used 100% (it can be mixed or reconstituted).
Drinking Water in particular is generally RO water with some minerals added for “taste” (Spring Water is generally fine if it is true spring water). Not that there is anything wrong with RO or DI water, it is just they are devoid of VERY important electrolytes and trace elements needed for proper fish respiration and osmotic function, without which you may be worse off in terms of fish health than with slightly polluted tap water.
So please use the information in this article to improve your water quality and make wise choices as to your water sources.
Please read this article about Aquarium electrolytes and more:
AQUARIUM CHEMISTRY; CALCIUM, KH, AND MAGNESIUM IN AQUARIUMS
Further Resources
- For a more in depth article about Aquarium Test Kits, please follow this link:
AQUARIUM TEST KITS; what they are used for and their importance - "Chlorine and the Reef Aquarium"
- Using Vitamin C To Neutralize Chlorine in Water Systems
Carl Strohmeyer copyright- 1/22/19
Other Recommended Reference & Product Sites

Aquarium or Pond UV Sterilization

Aquarium & Pond Information and Resources

Fish Diseases | How to Treat Sick Fish
*Fish Nutrition for Aquariums
TMC V2 RO Filter systems; the very best you can buy with TDS meter (far superior to 4 stage RO/DI systems sold via Bulk Reef Supply, Amazon, or eBay that use the inferior cellulose triacetate membrane made by Dow):
 Reverse Osmosis Aquarium Water Filters; with TDS Meter
Reverse Osmosis Aquarium Water Filters; with TDS Meter
*Aquarium Ich; Ichthyophthirius Multifilis and Cryptocaryon Irritans
*Complete Pond Care
*The Aquarium Nitrogen Cycle; COMPLETE
*Melafix Dangers? Bettas, Gouramis

UV Replacement Lamps/Bulbs
For TRUE High Output, Hot Cathode, Low Pressure UVC Germicidal Bulbs, not the low output medium pressure bulbs commonly sold at Amazon or eBay
ADVERTISEMENT
Labels: aquarium, Chloramines TDS, Chlorine, City Water, electrolytes, pond, sodium, Tap Water Conditioner, Total Dissolved Solids
<< Home







