Our Facebook Page to Follow: Aquarium/Pond Answers Facebook
This is a unique resource for answers, help, & advice to aquarium and pond questions not found elsewhere; With regular posts & article updates.
In our research; we use aquaculture, horticulture, medical, & university research to compile many of our articles.Our Recommended Lighting for highest efficiency professional planted/reef aquariums: "AquaRay Lighting"
Head Pressure in Aquarium and Pond Water Pumps/Filters
Sections Include:
- Overview
- Simple Calculation Methods
- Impeller Design effect on Head Pressure
- Effect of Electromagnet design on Pump Head Pressure
- Head Pressure Improvement Suggestions
By Carl Strohmeyer-PAMR 40+ years experience
Updated 11/1/21
An aspect of any water pump (not air pump), including the water pump aspect of an aquarium canister filter, a significant factor in choosing the correct pump is head pressure.
I will attempt to describe in basic and more advanced terms the important aspect of "mating" a correct water pump to your aquarium or pond application, including the addition of placing a UV Sterilizer in the line up.
The most simple definition of head pressure is that this is the force (or resistance) placed on the "head" (OUTLET) of the pump by gravity. The weight of the water column past the point of the pump outlet and devices in-line past this point constrict or impede flow. A UV Sterilizer, pond "spitter", fountain, waterfall feature, or Fluidized Filter all can affect head pressure.
Do not confuse this with the INPUT side of a pump, any resistance or variances in flow placed on the input side of a pump can damage a pump, especially the popular Mag-drive pumps used today by most aquarium & pond owners (in applications of possible input resistance, a direct drive pump should ONLY be used)
In other words the ability to LIFT water while maintaining current (think torque in a truck that allows the "lifting" or pulling of a load up a hill). This is not simply moving water on a level plain as flow, directly out of the water pump with no pressure placed on the pumps flow.
Another way to think of this is to take a 5 gallon bucket full of water. To simply tip and pour water out of the bucket takes very little energy or "lift" (as an example of head pressure). However, if you were to climb a ladder and pour this same bucket, it would take more energy or lift to do so (again as an example of head pressure).
The majority of aquarium and pond water pumps list their maximum head pressure, so this can be used to determine the end water flow (after head pressure is applied). This may be essential for not only determining the correct pump to purchase, but the correct UV Sterilizer (if desired) to be purchased since flow rate is a MAJOR FACTOR in UVC Sterilization effectiveness.
The listed "GPH or "LPH" is almost always the flow rate directly out of the pump (head) WITHOUT ANY pressure from tubing, devices, filter media, or even water or gravity applied.
An example/analogy would be a car/truck engine: the rated horsepower is what the engine produces at the crank shaft, not the wheels after transmission, vehicle weight, etc is applied. So as a rough analogy; a 200 hp engine is going to work much better in a 2000 lb vehicle than a 10,000 vehicle. This analogy applies to a pumps rated flow rate before head pressure is applied.
"Real World" Canister Filter Flow Rates:

A pump in a sump is an open system, where as a motor/pump as part of a canister filter is a closed system by virtue of the aid of a siphon. This Siphon aid also applies if we were to use a water pump placed in the aquarium to power the canister filter, which I have often done when the motor has failed (this is less expensive than a new filter and often makes for an easier starting filter).
This siphon aid results in much lower affects of distance upon water flow rate between a canister filter and a pump in an open sump.
What is noteworthy is that despite this siphon aid, tests and 1000s of practical use applications show that there is still a drop in flow rate for a closed system canister filter due to friction, tubing size, devices such as UV Sterilizers, CO2 equipment, etc. along with some impact from head pressure.
This sometimes confusing aspect also applies when one product is more factual in publishing flow rates with what a canister filter's closed system pump will deliver in a "real world" application than another.
An example here would be a Sunsun 303B Aquarium Canister Filter which like most related filters provides only the gph (370 gph) immediately at the "head" of the pump, while the Rena Filstar XPL provides both the immediate flow rate out of the pump head (350 gph) and the ACTUAL flow rate (187 gph) out of the filter itself after hoses, filter media, etc are applied.
Based on questions, many will think the SunSun and related filter is substantially stronger, when in reality these filter are nearly equal once head pressure is applied.
Recommended Product Sources:
*Sunsun 303B Aquarium Canister Filter
*Rena Filstar XPL Premium Canister Filter
The implications here are important for mating an effective UV Sterilizer to the correct filter (if a canister filter is to be used to drive water through the UV Sterilizer). As noted previously, few canister filters rate their "true" flow rate. Another example is the Fluval FX5 with a 0 head pressure rating 925 gph, when in reality the typical head pressure flow rate is 600 gph or less after the added resistance in the filter media and tubing are applied (as well as the addition of a UV Sterilizer).
Though it's far from an exact formula, a typical pump/canister filter flow rate with an under tank placement is about 50% to 60% of the published 0 head flow rate.
This is the number you should use for mating your UV Sterilizer.
It's noteworthy that you can increase or decrease the head pressure by the placement of the pump/canister filter and other equipment run off the filter. Keeping the tubing length to a minimum can help decrease friction & head pressure as well as to not use under sized inside dimension tubing.
Impeller, intake, internal flow, and exhaust design will also play a role, as I have seen one canister filter brand barely affected by placement, whereas another is much more affected by filter placement.
Here is an actual test using the Filstar S and timing its flow to fill a container.
- Level with the aquarium - 164.53 gph
- 24" below the aquarium - 153.00 gph
- 52" below the aquarium - 142.87 gph
These results were with a filter with NO resistance in the filter (all media was removed). The tubing was not cut, shortened or lengthened (as tubing length can also change results by adding more water resistance).
For example you can have an aquarium where the stand or table it is placed on allows for the filter and other equipment to be placed alongside at the same level as the base of the aquarium. This would provide for considerably more head pressure than an installation that has the canister filter and other equipment under the stand/cabinet at say 24 inches.
Another important plumbing note is that it is important that your inflow not be impeded (which is why a pre-filter is important, especially with pond pumps), if any flow restriction is needed to control flow, this should be on the exhaust/outlet side of any pump/filter system.
Usually valves on the output/outlet side only adds more head pressure, while restricting flow on the inlet side will strain a pump/filter (this is based on decades of practical/hands on experience as well as advice from my many mentors over the years).
* An example would be a pump with the ability to pump to 10 feet; there is no difference between running this pump at zero head pressure while restricting the flow rate to that of 5 feet of head pressure versus simply running the pump uphill 5 feet to an aquarium or pond water feature!
This is why most properly designed pumps or filter often have a larger diameter intake than they do exhaust. This allows for less strain on the impeller and better head pressure.
Water Pump Designs;
Most aquarium water pumps are open impeller designs that are greatly affected by head pressure. Propeller pumps, just by their design can handle almost no head pressure and thus are only intended for under water applications with no lifting of water out or into an aquarium.
A few pumps, in particular those designed for ponds or large aquarium systems such as the Rio HF Pump series have closed designs that can handle much more head pressure and are thus better suited for lifting water through multiple devices, water features, or deep sumps.
The bottom line is, regardless of the pump design you choose, know its maximum head pressure so it can be calculated what the actual flow will be with the devices you might add in-line.
For instance, do NOT expect a 1000 gph pump with a maximum head pressure of 10 feet to pump any more than 700 gallons per hours lifting water 3 feet from a pond to a waterfall (other factors including water in-line likely will reduce this even more). It is likly that it will be closer to 600-500 gallons an hour. This also applies to aquarium applications too!
Simple Calculation Methods:
This section will provide simple and reasonably (but not 100% accurate) methods to calculate head pressure on a pump or filter.
:
This one is the most obvious; Add 1 foot of head pressure for every 1 foot of vertical tubing past the pump or filter to the discharge point in the aquarium or pond. If the pump is submersible, do not count the position in the aquarium or pond, just where the tubing leaves the top level of the water.
Do not count tubing coming from an aquarium to a canister filter as this is gravity assisted and does not add to this calculation.
This major factor of head pressure is a "one way" factor, meaning that if your pump/filter is pumping water to a UV Sterilizer 24 inches under the aquarium water level, you do NOT count the distance down, then back up as "down" is a given based on gravity/siphon. The head pressure is added on the trip back up to the tank/aquarium.
Looking at Vertical Head Pressure another way we can use this accurate formula:
1 vertical foot = 0.433 pounds per square inch (psi)
or conversely
1 psi = 2.31 vertical feet
Look at it this way; if you have a sump, and are pumping the water up, you have atmospheric pressure at the intake and you have atmospheric pressure plus the weight of the water column on the output of the pump. So before even moving anything the pump has to overcome the weight of that column, and that's where all those feet and 2.3ft/psi or 0.433 psi come from.
As an example; A pump with a rated Head Pressure of 7 feet, would have 3.03 psi (7 divided by 2.31)
Another more simplified, albeit not 100% accurate way to figure vertical head pressure loss is to find the rated maximum head pressure of a pump or filter (if published), then deduct the amount of head pressure in feet, inches, centimeters, etc.
As an example; if the maximum head pressure is 6 feet, and you are placing a pump or filter 2 feet below the aquarium or running 2 feet of water line up to a fountain or pond water feature, you can roughly deduct 1/3 off the flow. If 3 feet of water line is used for this same pump/filter, you can roughly deduct 1/2 (50%) of the flow.
There are not 100% accurate formulas that I have found for measuring horizontal head pressure, other than the very complicated Bernoulli's Equation.
Reference: Bernoulli's Equation
However both in my observations and other reading it is safe to say that horizontal head pressure equals a vertical run of .25 to .5 times its length (in an open system).
The pump outlet size is a major determining factor that you can calculate; for instance if you have a pump with an outlet designed for 1 inch tubing and you use a reducing part (often supplied with many pumps) for say 1/2 inch, you are literally going to reduce your total head pressure by half.
However if your pump is designed for only 1/2 ID tubing, using this size tubing/pipe is not going to subtract from calculation.
I also should point out that not all pumps are designed with the best outlet sizes for their design (which includes head pressure, impeller design, and more), so these calculations can be off if for instance a pump is designed with a 1 inch outlet when in reality its design is better suited for 3/4 inch outlets.
As a side note, in tests I have conducted with several pumps and 1/2 ID tubing with one foot of head added I have not been able to achieve flow rates beyond 350 gph regardless of open impeller pump sizes, wattages, stated flow rates, etc.
Many aquarium systems are designed to operate with larger diameter tubing/piping on the inlet size and smaller on the outlet.
This reduced friction and allows for better flow rate and less strain on the pump/motor.
This also applies with both closed and open systems which is why pumps such as the Rio HF pumps have a larger diameter inlet than outlet
Further Reference:
Will a Larger Pipe Size Help or Hurt?
The bottom line is this is an aspect I have often seen where clients/customers I have serviced over the years have over looked.
An example was a person who had a 3800 gph pond pump connected to 1 inch tubing, even though the pump was designed for 1.5 tubing/pipe. This reduced the flow immediately by 1/3!!
Generally speaking a Level 1 UV Sterilizer will add 1-2 feet to head pressure, but this can vary considerably by UV size and design.
As an example a small well designed 8 watt UV would add as little as a foot to head pressure, however some, such as the Coralife 9 Watt Turbo Twist can double this for a similar size UV.
A large UV such as the TMC 110 Watt PRO Pond /Aquarium UV will add as much as 4 feet of head pressure (although less if 2" ID inch pipe is used).
Product Resource:
*Level 1 UV Sterilizers
*TMC 8 Watt Vecton Premium UV Sterilizer
*TMC 110 Watt PRO Pond /Aquarium UV Sterilizer
Devices such a Fluidized Filters can add anywhere from 2 to 4 feet of head pressure (more or less).
Again design is a factor as well as the sand size (smaller requires less head pressure), but again as a generalization consider a minimum 1 foot for every 6 inches of sand fluidized plus the added elbows in the device add to head pressure as noted earlier in section #2.
Product Resource: TMC Premium 3rd Generation Aquarium Fluidized Sand Bed Filter
If this cannot be found, find a similar pump with the same input wattage and one can generally extrapolate what the maximum head pressure will be.
Then use this number for calculations of flow rate. Using an example is the best way I can explain this:
If, for example your pump/filter has a maximum head pressure of 8 feet and your device (such as a UV Sterilizer) is one foot below the aquarium or pond, you have one 90 degree bend in 3/4" ID tubing/PVC (this would add 1/2 foot head), and then the device itself would add a minimum of one foot head, this equals 2.5 feet of head pressure. Then divide 2.5 feet by 8 feet and gives you .31 or 31%.
So if the rated flow rate of said pump or filter is 500 gph without any head pressure (rated flow), then multiply by .31 or take off 31%, this equals 156 gph. Then deduct 156 from 500 and your actual flow should be 344 gph.
If all else fails in calculations or you have already purchased a pump and simply desire to know the end flow rate, this can be simply calculated quite exactly;
Simply place a container under the outlet of your pump as it empties into the aquarium or pond after passing through all devices and water feature. Then time how long it takes to fill the container in exact measurements.
As an example if a 1 gallon container fills in 10 seconds, that is 6 gallons per minute or 360 gph (this works for metric using liters too).
Admittedly this can be rather difficult for very large flow rate pond pumps that pump say 10 times the previous examples flow rate (3600 gph versus 360 gph), as this would fill 1 gallon in a second. This would required a 5 gallon bucket that might be difficult to position 100% in the water flow discharge; the end result would be 5 gallons in 5 seconds to produce 3600 gph.

Video; Calculating Aquarium Flow Turn Over
ADVERTISEMENT
Effect of Impeller design on Pump Head Pressure
The design a of a water pumps impeller has a major impact on whether it will produce higher flow rates through a higher flow rate curve.
Here are three common designs and their affect on water head pressure
(Please Click on the pictures to enlarge);
 The "Propeller" pump impeller design has almost no head pressure and is not intended for any real lifting of water.
The "Propeller" pump impeller design has almost no head pressure and is not intended for any real lifting of water.
However it also is very efficient at moving reasonably high volumes of water with a very smooth rotating current and requires very low electrical wattages to do so.
These are popular in pumps such as the Premium Seio Propeller pumps for under water circulation devices, especially in reef tanks.
Another advantage of this impeller design is that it also has very low start up resistance making it the best design for wave makers that automatically turn pumps on and off to simulate waves.
Product Source: Seio 320, 530 Propeller Water Pumps for Reef Tanks, wave makers
 The standard open impeller is the most common design for aquarium and light duty pond water pumps.
The standard open impeller is the most common design for aquarium and light duty pond water pumps.
While not as good for wave makers, these tend to be the most versatile design which use reasonably low electrical wattage.
Even with these impellers there is a very wide variance in design quality with some having lighter magnets, thin blades or even slight hybrid propeller designs (the Fluval Pumps have impeller designs that are not well suited for adding much head pressure).
As pure water pumps/ power heads go, the newer upgraded Rio Plus Pumps are the superior design in this class from what I have used and seen.
Product Resources:
*Rio Aquarium, Fountain Water Pumps
*Aquarium, Fountain, Pond Pumps; Light to Heavy Duty
 The "closed impeller" design is generally the most heavy duty water pump impeller.
The "closed impeller" design is generally the most heavy duty water pump impeller.
This design can handle many more in line devices and its head pressure falls off much more slowly towards its maximum head pressure (where it obviously falls off to 0). In fact this is an aspect of pumps that utilize these closed impellers that is difficult to measure in the previous sections calculations (which I made many generalizations).
This design is best for deep sumps, multiple water features/devices, multiple aquarium systems, and simply larger ponds or aquariums.
A good example of a well made closed impeller pump is the Rio HF Pump
Product Resource: Rio HF Pump; Medium to Heavy Duty Water Pumps
Affect of Electromagnet design on Pump Head Pressure
This is rather straight forward, but still occasionally missed by aquarium or especially pond keepers, and that is the design of the electromagnet of most typical "Mag-Drive" pumps used for aquariums and ponds.
One way to think of this in terms of car/truck engines is horse power versus torque.
Many simple pumps (or in the case of high flow pond pumps; "cheap") have the horse power to move a lot of water, but almost no torque to lift water if there are any devices in-line or debris in the water.
The electromagnet that drives a propeller pump is generally very "simple", meaning it does not need to be a very heavy winding that uses much current, thus this design is generally quite efficient as for electrical usage. As an example a Seio 530 Pump uses only 7.5 watts yet moves 530 gph.
This is an excellent pump for what it is designed for, but it has absolutely no torque and therefore should be used for nothing more than underwater current, not running a UV Sterilizer or similar.
Product Resource: Seio 530 Pump
With most standard impeller design and especially closed (or partially closed) impeller design pumps the electromagnet is much heavier and will require more electrical current (wattage). This will obviously increase depending upon the load and flow it is designed for.
Unfortunately not all pumps marketed for applications such as ponds in particular have the heavy duty electromagnet to provide the torque to provide the "lift" necessary for head pressure.
So even if the flow is good or even the impeller design is excellent, often the electromagnet is not, so the end result is often poor head pressure or worse; a shorter life span due to an electromagnet burn out from attempting to run a large pump and impeller design (often in harsh pond conditions) with an inadequate electromagnet design.
A good example is the Via Aqua 4900 pump (although I like many Via Aqua pumps as good, albeit more economy pumps, this is one to be avoided).
Another is the entire Laguna Max-Flo pump line.
Often, but not always, a dead give away is a pump of what may seem "too low of wattage" for a high flow pump.
This may be fine a for a propeller pump, but not a pump used in sumps or ponds to lift water, especially with more debris in the water column or devices in-line.
Head Pressure Improvement Suggestions
This most basic suggestion is to adjust the level of lift if head pressure (slow water flow) is a problem.
As an example, say you are using an Internal Filter or Power Head Pump to run a UV Sterilizer (often utilizing intake and return adapters) such as the Vecton 8 Watt UV; I would recommend hanging the UV Sterilizer just below the rim of the aquarium with minimal tubing between the UV Sterilizer and the intake and return connections.
Such a short drop will reduce head pressure considerably versus placing the UV Sterilizer at the base of the aquarium or even lower such as at the base of an aquarium cabinet (which may be too much head pressure for many small Internal Filters and Power Head Pumps).
Product Resources:
*Via Aqua or SunSun Internal Aquarium Power Filters
*Power Head Pump; Efficient JP-023
The above suggestions work for many similar applications, and that is moving any device closer to the aquarium (or pond) thus reducing vertical lift.
Horizontal lift is also and issue, but not nearly as much so as vertical lift; that said reducing any unnecessary horizontal tubing/piping can improve head pressure as well.
Having direct flow, even with canister filters using bulkheads can also lower head pressure from my experience
This video provides information as to bulkhead use based on professional experience:

Aquarium Bulkhead Tips and Tricks
References, Additional Reading:
*Pipe Pressure Loss Calculator
This is excellent reading for those with a more technical engineering aptitude.
````````````````````````
For other articles to help readers make well informed decisions about their aquariums or ponds, please consider reading these:
*Aquarium Lighting; The most in depth & researched aquarium lighting article anywhere on the internet.
*UV Sterilization; Sterilizer Information
As with the Aquarium Lighting, this UV Sterilization article is a must read for aquarium or pond keepers.
*Pond Care Information;
Basic but complete information about pond care with links/resources to more in depth pond care help/information.
Other Recommended Reference & Product Sites

Sponge Filtration; Complete Aqurium Sponge Filter Use Information

Premium Aquarium Sponge Filters; from AAP/ATI

Aquarium & Pond Plumbing Parts
Difficult to find or unique parts found nowhere else!
Including: T Water Diverters, Hose Barb Adapters, Couplings, & Reducers, Ball Valves, Swing Check Valves, Return Adapters, Intake Adapters

Economy Submersible Aquarium, Fountain Pumps; SunSun JP-033
A better, UPDATED version of the Via Aqua 302 with SUPERIOR Performance, unlike other pumps sold elsewhere as a replacement

AAP/SunSun HJ-1542 Aquarium Pump; replaces Via Aqua 1300
This pump replaces the Via Aqua 1300 and other copies such as by AquaTop as the Premier Power Head Pump for Aquariums, Ponds, Fountains, Wet/Dry Filters. This pump is submersible with Mag drive & ceramic shaft

JTP-12000 High Output/Efficiency Pond Pump, 3170 GPH (12000 LPH)
Unique VERY efficient design; uses variable frequency technology for low power consumption yet large flow rate & high head pressure!
Uses only 100 watts for 3170 gph output

Hanna Instruments Aquarium Testers & Meters; For Freshwater and Saltwater
Hanna instruments is a global manufacturer of analytical instrumentation.
Hanna offers multi-parameter bench top portable meters and testers..

AAP Premium FSB Filters
Premium, second to NONE Aquarium Bio Filters, that with optional Oolitic Sand can also maintain essential aquarium calcium levels, alkalinity, & electrolytes that are important to ALL Marine life, Goldfish, African Cichlids, Livebearers & more
*Aquarium Silicone Sealant; USDA 100% Fish Safe
100% Fish Safe, USDA & Agricultre Canada approved.
The same CANNOT be said for Hardware Store brands!!
For a friendly, Knowledgeable, aquarium forum with in a family atmosphere:
Aquarium Forum; Everything Aquatic
ADVERTISEMENT
Labels: aquarium, Aquarium Pond Answers Directory, Aquarium Pump, Fountain, Head Pressure, Pond Pump, UV Sterilizer, Water Feature, Water Pump
Activated Carbon for Aquarium or Pond Use; Information, Use Table
Use of Activated Carbon in Freshwater, Marine Aquariums and Ponds
Index:
- How Activated Carbon Works
- Common Carbon Uses
- Lignite Activated Carbon
- Chemical Properties
- Contaminant Properties
- Water Temperature and pH
- Exposure Time
- Possible Concerns with Carbon Use
- List of compounds carbon can or cannot absorb
By Carl Strohmeyer-PAMR 40+ years experience
Updated 1/23/20
 Carbon is primarily an adsorbent which is a very popular chemical filter media that is often misunderstood as to use in established aquariums and ponds as well.
Carbon is primarily an adsorbent which is a very popular chemical filter media that is often misunderstood as to use in established aquariums and ponds as well.
A healthy established aquarium (fresh or salt) with regular water changes generally needs little carbon (although more carbon is generally needed in marine reef aquariums and less in low pH freshwater aquariums).
Carbon will NOT remove or absorb ammonia, nitrites, or nitrates. Carbon is very useful in removing medications after treatment or even between doses.
Please read the entire article for a good understanding of what activated carbon can or cannot do for your aquarium/pond; including the table of what carbon can and cannot remove as well as the referenced resources
How Activated Carbon Works
Activated carbon has an extremely large surface area per unit weight, which makes AC an extremely efficient absorptive AND adsorptive material.
The activation of carbon and its manufacture create many pores within the particles, and it is the vast areas of the walls within these pores that account for most of the total surface area of the carbon.
In water, activated carbon has a preference for large organic molecules and for substances which are non-polar in nature.
The forces of attraction between the carbon and the absorbed molecules are greater the closer the molecules are in size to the pores. The best absorption takes place when the pores are just large enough to admit the molecules.
Activated carbon, when contacted with water containing organic material, will remove these compounds selectively by a combination of adsorption of the less polar molecules, absorption (filtration) of the larger particles, and partial deposition of colloidal material on the exterior surface of the activated carbon.
As well as absorption, activated carbon uses a process called Adsorption, in fact adsorption is the primary method of molecule removal by carbon, not absorption.
When a material adsorbs something, it means it attaches it by chemical attraction.
The extent of removal of soluble organics by absorption depends on the diffusion of the particle to the external surface of the carbon and diffusion within the porous adsorbent. For colloidal particles, internal diffusion is relatively unimportant because of particle size.
Organic substances that pass through the column consist of hydrophilic organic molecules (substances that are attracted to, and dissolve well within, water) and hydrophobic molecules (repulsed by water).
If the molecule is “polar” (having both a hydrophobic and hydrophilic attributes) which many organic molecules are, the hydrophobic side will be attracted (attached) to the activated carbon.
Adsorption is partially the result of forces of attraction at the surface of a particle that cause soluble organic materials to adhere to the activated carbon. The limited water solubility of many organic substances will affect AC adsorption of these molecules.
Put more simply (I hope): Polar, hydrophobic and hydrophilic interactions are important interactions necessary to understand how activated carbon adsorb the certain molecules.
A Methylene Blue dye molecule is hydrophobic and has a large affinity to the hydrophobic carbon rings of the activated carbon.
The dye prefers to interact with the carbon rather than water.
Where as non chelated metals (such as copper ions) are positively charged (hydrophilic), and the carbon is neutral and hydrophobic. Therefore, the positively charged metal ions prefer to interact with the water, which is hydrophilic. “Like dissolves Like”.
This applies to most metals from the periodic table, including calcium, magnesium, etc., and for this reason most essential minerals are not removed by activated carbon unless chelated but they can loose their positive charge due to oxidative processes of the Redox Potential, which is another reason to replenish these ESSENTIAL positive mineral ions, especially if carbon is used.
This positive/negative ionization is why DOC (organics) will also negatively affect the Aquarium/Pond Redox Balance
Please see this article for further information:
The Aquarium Redox Potential
ADVERTISEMENT
Common Uses
As already noted healthy aquariums often do not need much activated carbon, however this is a rather ambiguous generalization.
An aquarium keeper needs to consider DOC (dissolved organic compounds/carbon), pH, desired compounds that can or cannot be removed, bio load of the aquarium or pond, and desired environment.
As an example, an Amazon River aquarium will generally need less or no carbon while a heavily fed reef tank will need more.
One reason for little or no carbon in an Amazon River aquarium is these are generally low pH environments that are high oxidizing with little or no Redox Reduction. As noted earlier, positively charged metal ions once they give up their positive charge are easily removed and in a low pH, highly oxidizing aquarium this can be done rapidly. The use of Carbon, especially in larger quantities will only speed this process.
I have made many tests and observations over the years in freshwater and marine aquariums of different environments, as well as ponds as to how much carbon is best to use (if any).
I would use tests of pH/KH and nitrates to help determine need. Although carbon cannot remove nitrates, it can remove most DOC that will eventually end up as nitrates.
So a falling pH/KH and climbing Nitrate level would generally be an indicator more activated carbon or some carbon is needed.
Redox tests if available can sometimes be helpful since activated carbon can act as a Redox Mediator.
See this article for further reference:
Redox as it Pertains to Aquariums; Including Methylene Blue Test
Product Source: Methylene Blue
Simply gauging activated carbon use by tank water color, if the tank is yellowing this can be an indicator of the need for more carbon. However this is not a 100% accurate indicator, nor can activated carbon remove all the causes of yellow water.
The use of carbon if only for a day or even hours after or between medication treatments is another important aspect of carbon use.
In Reef aquariums, the use of carbon (even though less effective in higher pH water) is important in my experience/opinion as part of a regimen that where carbon is but one piece of the aquarium maintenance puzzle that often includes Protein Skimmers (which also remove many similar DOC, but not as quickly after production), water changes, micron filtration, de-nitrification with deep sand beds or products such as Matrix, and use of chemical absorbents such as Purigen.
Product Sources:
*TMC V2 Premium Marine Aquarium Protein Skimmer
*SeaChem Matrix
*SeaChem Purigen premium synthetic absorbent

Carbon can also be used in mixed products such as Ammo Carb, but zeolites are only for freshwater use.
The use of zeolite/carbon mixes is especially useful in areas where municipal tap water contains chloramines (instead of the usual chlorine).
I have also achieved good results with carbon/zeolite blends in ponds which are generally exposed to even more contaminants than aquarium.
Further Information: Municipal Tap Water, Chloramines
Product Source: Ammo-Carb from AAP
A good starting point for activated carbon use (please note that this is a generalization), is one to three teaspoons per ten gallons of water.
One teaspoon of activated carbon is equal to approximately 6 grams in weight measurement. This amount will vary greatly depending upon many other factors that the aquarium or pond keeper can determine.
As well sometimes an aquarium keeper may choose to only use this amount of carbon as part of a clean up procedure (in particular freshwater aquariums) and then discontinue use after a day or two.
Lignite Activated Carbons
 The best Premium Activated Carbon is produced from a soft, brownish-black coal (Lignite) in which the alteration of vegetable matter which has proceeded further than in peat but not as far as in bituminous coal.
The best Premium Activated Carbon is produced from a soft, brownish-black coal (Lignite) in which the alteration of vegetable matter which has proceeded further than in peat but not as far as in bituminous coal.
Lignite based carbon is the best choice for use in aquarium & ponds to remove organic molecules, pesticides and for color removal, due to its large pore size.
Product Source: High Grade Lignite Pelletized Carbon from AAP
The medium to large pore size is important, because the organics in a aquarium or pond environment will clog and render ineffective, the smaller-pored, coconut shell carbons.
6 grams (.21 ounces) of Lignite Activated Carbon has the surface area of a football field, so a little goes a long way in aquarium use in particular.
The other common coal based carbon are the Bituminous coal activated carbons. These are harder with more varied pore sizes.
The graph below compares some of the coal based carbon properties:
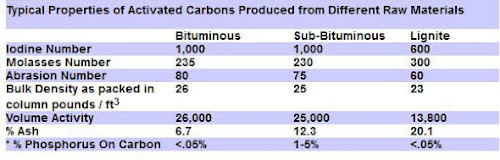
Here are a few aspects that impact the effectiveness of activated carbon
Chemical Properties;
The carbon surface may actually interact chemically with organic molecules. As well electrical forces between the activated carbon surface and some contaminants may result in adsorption or ion exchange.
Adsorption, then, is also affected by the chemical nature of the adsorbing surface. The chemical properties of the adsorbing surface are determined to a large extent by the activation process.
Activated Carbon formed from different activation processes will have chemical properties that make them more or less attractive to various contaminants.
Contaminant Properties:
Large dissolved organic compounds/carbon (DOC) are most effectively adsorbed by activated carbon. A general rule of thumb is that similar materials tend to associate. DOC molecules and activated carbon are similar materials; therefore there is a stronger tendency for most organic chemicals to associate with the activated carbon in the filter rather than staying dissolved in a dissimilar material like water.
Generally, the least soluble organic molecules (such as large complex amino acids or fatty acids) are most strongly adsorbed. Often the smaller organic molecules (such as sugars) are held the tightest, because they fit into the smaller pores.
It is also noteworthy that although larger complex organic molecules (often nitrogen based) are more readily absorbed, these molecules are also not held as tightly and re-leased under certain conditions which is why carbon should not be relied on for the sole form of organic contaminant removal.
Other methods such as Purigen, de-nitrifying filters, water changes, Protein Skimmers (marine aquariums), micron filters, UV Sterilizers, etc. should be employed as part of the mix.
Concentration of organic contaminants can affect the adsorption process. A given activated carbon may be more effective than another type of activated carbon material at low contaminant concentrations, but may be less effective than the other carbon material at high concentrations.
Water Temperature and pH;
Adsorption usually increases as pH and temperature decrease. Chemical reactions and forms of chemicals are closely related to pH and temperature. When pH and temperature are lowered many organic chemicals are in a more absorbable form (this is noteworthy for marine/saltwater use and why Protein Skimmers are also important as these devices will remove DOC as well, although not immediately as carbon can)
Exposure Time;
The process of adsorption is also influenced by the length of time that the carbon is in contact with the contaminant in the water. Increasing contact time allows greater amounts of contaminant to be removed from the water. Contact is improved by increasing the amount of activated carbon in the filter and reducing the flow rate of water through the filter.
There is controversy in what essential minerals carbon will absorb or what activated carbon will or will not absorb in general. I will state based on my own experience and scientific evidence that carbon has many uses in aquariums/ponds but is also over used or incorrectly recommended. Although I use little carbon in my established healthy aquariums and ponds, I disagree with those that state it should not or rarely be used (based on some false assumptions of what carbon removes or adds to water).
On the flip side I also disagree with those that make carbon the answer for water quality issues such as nitrates for which carbon does not remove.
Activated Carbon is very useful for removing most medications after or between treatments (this is where I strongly recommend its use), although even here, carbon does not remove most copper formulations effectively.
Possible Concerns with Carbon Use
*Activated carbon can foster the growth of bacteria by concentrating other organics (such as DOC) on its surface.
Although I have not performed controlled tests to confirm this, I have made many observations over the years that increased use of carbon has coincided with increased incidence of bacterial infections such as Aeromonas, especially in lower pH Amazon River & SE Asia water aquarium environments since Redox is also generally less favorable and the removal of tannins by carbon from products such as Indian Almond Leaf Extracts allows these bacteria to thrive.
The fact that activated carbon removes oxygen, further increases the risk of an opportunistic Aeromonas Bacterial outbreak since these bacterium are anaerobic.
Further References:
*Aeromonas, Septicemia, Vibrio Infections in Aquarium Fish
*Aquarium Chemistry; Amazon River Water Environments
Product Source: Atison's Spa, Indian Almond Leaf Extracts, lowers Aeromonas
Use in planted freshwater aquariums:
This is an area of some controversy of which some information is based on facts, some information is not, with some reasonable questions in between.
The main controversy I will address for now as to carbon use in planted freshwater aquariums is the removal of trace minerals. I read some experiments at “the Krib”, as well I have made observations and tests (as well as research) over the years myself.
The main testable point is that most metals such as Iron (which is important for plants) are NOT absorbed carbon with an important and noteworthy exception; and that is the use of chelation.
EDTA (which is an organic molecule) is used to chelate many metals such as iron to make it more readily available for fertilizers or other uses, and since activated carbon is especially effective in removing organic carbon based molecules, these chelated metals are then removed.
Any aquatic plant fertilizers that contain chelated metals will be bound to the carbon pores, and as result their concentration into the water column will get lower with the use of activated carbon.
If the carbon is left in the aquarium for a period of time, the chelated compounds in aquariums slowly decay and release their metals.
However not all trace elements are chelated, for instance SeaChem Flourish uses water soluble non-chelated iron, as well mineral blocks such as Wonder Shells are non chelated and any possible absorbed trace minerals are rapidly replaced by the Wonder Shell (which although mineral depletion by activated carbon is low, the use of Wonder Shells in aquariums/ponds utilizing activated carbon insures adequate minerals)
Product Sources:
*SeaChem Flourish Plant Fertilizer
*Wonder Shells; Unique Version ONLY available at AAP
The use of Activated Carbon with Marine Protein Skimmers:
Although conclusive tests are forth coming, there is evidence that the use of activated carbon can limit the amount of foam refraction generated by a marine protein skimmer. This is likely due to the adsorption of Foaming Agents (MBAS) by activated carbon.
This presents a problem for many reef keepers since both carbon and protein skimmers are useful aspects of a complete marine filtration system.
My suggestion is to limit carbon use in cleaning filters run during certain times of the day (or week), especially after heavy feeding.
The re-use of carbon after removal during disease treatment:
Carbon can trap many pathogens, so soaking in saltwater (specific gravity of 1.025) while out of the aquarium can prevent re-infection once added back into the aquarium.
HOWEVER, this is far from 100% sure, especially with viruses, and some bacteria and certain fungi (generally the saltwater soak is very effective to kill parasites).
Soaking in bleach/chlorine or any other Redox oxidizer is not a viable alternative since (as per the table below), carbon is very good at removing these products. What will happen is these will cancel each other out; either the amount of bleach or similar used will exhaust the carbons capability or the carbon will remove all the oxidizer this not allowing any disinfection.
*A final concern with activated carbon is the possible release of contaminants after they have been initially adsorbed. This action is known as desorption or dumping. This could occur if other ambient water quality characteristics change.
Although at the time of writing this article, I have not discovered the exact mechanism for causing this, but I do know that a tank that is not stable in its general chemistry, whether pH or Redox is a candidate for this possible problem of carbon use.
Here is a list of compounds carbon can or cannot absorb.
Please note that some compounds may be desirable to remove depending upon your tank requirements while the same compound may not be desirable in another aquarium environment.
A good example would be tannins (which carbon removes reasonably well); in a soft water environment an aquarist would likely want to restrict the use of Activated Carbon, while someone keeping “dirty” goldfish may find the use of carbon on a regular basis (in higher quantities) a necessity.
| WHAT CARBON CAN ABSORB: | ||
|---|---|---|
| Excellent Absorption: | Fair/Good Absorption | WHAT CARBON CANNOT ABSORB (or absorption is poor) |
| *Amyl Acetate *Amyl Alcohol *Benzene *Bleach *Butyl Alcohol *Butyl Acetate *Calcium Hypochlorite *ORGANIC carbon *Chloral *Chloroform *Chlorine *Chlorobenzene *Chlorophenol *Cresol *Defoliants *Diesel Fuel *Dissolved Organic Compounds *Dyes (such as Methylene Blue or Acriflavin) *Ethyl Acetate *Ethyl Acrylate *Foaming Agents (MBAS) *Gasoline *Glycols *Herbicides *Hydrogen Peroxide *Hypochlorous Acid *Insecticides *Iodine *Isopropyl Acetate *Isopropyl Alcohol *Ketones *Methyl Bromide *Methyl Ethyl Ketone *Naptha *Nitrobenzene *Nitroluene *Odors (general) *Oil - dissolved *Organic Esters *Oxalic Acid *Oxygen *PCB's *Pesticides *Phenol *Sodium Hypochlorite *Toluidine *Trichlorethylene *Turpentine *Xylene | *Acetaldehde *Acetone *Alcohols *Antifreeze *Chloramine (only the Chlorine, zeolite needed for remaining ammonia) *Calcium Hypochlorite *Chlorophyll *Citric Acid *EDTA (an organic chelator of metals such as iron) *Ethyl Alcohol *Ethyl Amine *Ethyl Chloride *Etyl Ether *Hydrogen Sulfide *Lactic Acid *Mercaptans *Methyl Acetate *Methyl Alcohol *Methyl Chloride *Organic Acids *Organic Salts *Ozone *Potassium Permanganate *Propioc Acid *Propyl Acetate *Propyl Alcohol *Propyl Chloride *Radon *Solvents *Sulphonated Oils *Tannins, such as: Indian Almond Leaf Extract (Atison's Spa) *Tar Emulsion *Tartaric Acid *Xanthophyll *Cyanide *Copper (this in part depends upon type of copper compound and chelated copper is not readily removed by carbon), also availability of oxygen and a lower pH can improve basic copper sulfate adsorption by carbon. Basically carbon should not be relied for total copper removal, especially in Marine aquariums using chelated carbon *Free, low molecular weight hormones. | *Alkalinity *Calcium *Carbon Dioxide *Fluoride, *Formaldehyde *Hardness. *Lime *Magnesium *Manganese *Microbes, *Molybdenum *Nitrates, nitrites, ammonia *Phosphates *Selenium *Sodium, *Lead, Iron and other heavy metals are removed only by adding a chelation process using EDTA, an organic carbon molecule (then these metals can be readily removed) *Protein bound hormones are generally NOT removed by carbon |
SUMMARY;
It is noteworthy that not all carbons are the same, but hopefully the reader will understand what a quality activated carbon can and cannot do.
There are many excellent carbons available on the market, as well as many very poor carbons/charcoals.
My advice is if the carbon you are currently using is not matching the results expected by the above chart, then switch.
As well, do not feel stuck using manufacturer pre-filled carbon bags that are commonly sold for many filters. My experience has been that the majority of these are of poor quality, and both results and cost savings can be achieved by purchasing a separate carbon and refillable bag to use in your filter.
Product Examples:
High Grade Lignite Pelletized Carbon from AAP
Lees Filter Bags
References
*The Krib; Activated Carbon
*Copper & Cyanide Removal by Carbon
Other Recommended Reference & Product Sites

Aquarium Lighting, Information
The largest data base of aquarium lighting information available on the Web, which with Googles latest updates, good information is nearly impossible to find.

Fish Treatments, How They Work, Which to Use and Not to Use

NilocG Aquatics; Planted Tank Liquid Ferts from AAP
NilocG Aquatics PROFESSIONAL GRADE planted aquarium liquid fertilizer products.
Products designed for persons who want to have a more advanced planted aquarium without the hassles need for a degree in science to do so. Meant for use in "The Estimative Index of Dosing, or No Need for Test Kits" method of planted aquarium aquascaping.

Eheim Everyday Automatic Feeder
The EHEIM Everyday Fish Feeder is a compact fish feeder with actively aerated feeding chamber.
This unit includes a clamp for fixing to open top aquariums or terrariums. Easy to understand programming, double dosage of food is programmable
TMC V2 RO Filter systems; the very best you can buy with TDS meter:
 Reverse Osmosis Aquarium Water Filters; with TDS Meter
Reverse Osmosis Aquarium Water Filters; with TDS Meter
A good compliment to RO water or for any freshwater aquarium to add ESSENTIAL Mineral Ions:
*Wonder Shells, Mineral Block
Aquarium Forum; Everything Aquatic
An excellent place to go for information, help or simply to share your love of the aquarium and pond hobby and help others. A superior place for information over such places as Yahoo Answers

UV Replacement Lamps/Bulbs
For TRUE High Output, Hot Cathode, Low Pressure UVC Germicidal Bulbs, not the low output medium pressure bulbs commonly sold at Amazon or eBay
Planaria & Detritus Worms in Aquarium
Melafix Dangers; The Known Facts
Celestial Pearl Danio, Galaxy Rasboras
AAP/SeaChem Tidal High Capacity
Aquarium HOB Power Filter (By Sicce)
Premium "Hang On-the Back" Filtration Systems; Including skimmer feature & high capacity filter basket

Spirulina 20 Fish Food Flake
The best balanced fish flake food diet for Tetras and other fish for disease prevention
ADVERTISEMENT
Labels: Activated Carbon, aquarium, Aquarium Carbon, Carbon, Chemical Filter Media, Chemical Filtration, clear water, yellow water
Aquarium Nitrates; Lowering Nitrate Levels, How to Control
By Carl Strohmeyer-PAMR 40+ years experience
Updated 9/6/23
AQUARIUM OR POND NITRATES
Including:
Although much less toxic than ammonia and nitrite; Nitrate (NO3) as a nitrogen compound can also cause stress making a fish’s organs work harder to adjust to their environment, especially at levels higher than 100 ppm in many fish.
The increasing stress results in the loss of ability to fight diseases, the ability to heal itself, and the ability to reproduce.
It is essential for you, the aquarium (or pond) hobbyist, to maintain a proper environment for your aquatic companions. High nitrate levels are often a sign of poorly maintained aquariums and will cause problems in the long term.

How to Reduce Aquarium Nitrates Video
As a GENERALIZATION (which is the general consensus among experienced fish keeping sources, but also not totally agreed upon), I recommend maximum levels UNDER 40-50 ppm for FW (shooting for numbers under 10-30 for more optimum conditions for many inhabitants).
20 ppm or less for Saltwater fish, & under 5 ppm or less for reef aquariums. For planted freshwater aquariums, about 15 ppm is suggested (or even higher, as too low in planted aquariums can be a problem).
It is also noteworthy that very low nitrate levels in an established aquarium does not necessarily mean that the aquarium is not cycled.
As often a low bio load and/or aspects of natural nitrate reduction such as plants (or Pothos), refugiums, anaerobic de-nitrification filters, etc. along with nitrate lowering methods such as Algone, NPX Bioplastics, Protein Skimmers, etc. can IN FACT keep nitrates at near ZERO numbers. I've seen this 100s of times in my career with my client's aquariums.
If levels exceed these generalized numbers for a LONG period, NOT just a day or even a week, coupled with other water parameter factors such as poor Redox balance, over crowding, poor feeding, and more, this can and will affect long term fish health.
Further Reference: Aquarium Redox
 Long term high nitrates are potentially dangerous due to the effects on the water chemistry and on a healthy environment for your fish while nitrates are accumulating.
Long term high nitrates are potentially dangerous due to the effects on the water chemistry and on a healthy environment for your fish while nitrates are accumulating.
The higher the nitrate levels, the higher and more severe the consequences due to the stress on your fish and the favorable conditions for a serious algae outbreak.
The higher the the long term nitrate concentration the more stress for the fish. More severe stress is reached at levels exceeding 100 ppm.
Additional stress MAY be added due to an accumulation of life forms such as detritus worms feeding on decomposing waste, and the consequently higher biomass (organisms living in the aquarium) leads to an increasing demand of oxygen.
Reference: Aquarium Planaria? Actually Detritus Worms
I will also add that although nitrates are not dangerous in the short term unlike ammonia or nitrites; in established tanks I usually test this parameter more often as this is a good indicator of how well I am doing in my tank cleanings.
Example; if enough water is being changed and with the correct frequency, as well as to whether additional nitrate removing products or procedures need to be employed. Tests of KH & GH are also useful in indicating an established tanks health.
Reference: Aquarium Cleaning; Reasons, Frequency, more
One more point about nitrate; I have tested the water on under sized aquariums/bowls containing otherwise healthy goldfish.
The nitrates would often exceed 200 ppm! These goldfish (although they appeared healthy), rarely lived more than 3-5 years as compared to the 12 + years of the goldfish I have kept for clients in pond and larger aquariums.
While not fish, University studies in Cattle show nitrate levels in water over 221 ppm to be harmful or even fatal, so I sure would NOT want my fish kept at nitrate levels approaching this number.
Reference: Aquarium Cleaning
ADVERTISEMENT
Nitrates (NO3) are compounds composed of a nitrogen and three oxygen atoms and are often the final stage in the nitrogen cycle of fresh and saltwater aquariums if there are not nitrate removing plants, algae, or nitrate reducing anaerobic bacteria present.
Nitrates are the conjugate base (chemical substance that releases a proton in the backward chemical reaction) of nitric acid (HNO3), consisting of one central nitrogen atom surrounded by three identical oxygen atoms.
The presence or production of large amounts nitrates can result in the presence of Nitric acid according to the Brønsted-Lowry theory of acids and bases which will in turn affect an aquariums pH and KH (which can result in dangerous pH swings).
This will also have an affect on your aquariums Redox balance and likely result in higher oxidative stress on your aquarium's inhabitants.
For more about the nitrogen cycle, please reference:
*Aquarium Nitrogen Cycle
 A high Bio-Load that often produces large amounts of organic mulm and decomposition in an aquarium (or pond) gravel or in filters is often a common cause of persistent nitrate problems.
A high Bio-Load that often produces large amounts of organic mulm and decomposition in an aquarium (or pond) gravel or in filters is often a common cause of persistent nitrate problems.
Another clue to this is a pH that tends to drop quickly, often even with buffers added (assuming a higher new water pH); the breakdown of organic mulm or similar will lower pH while increasing nitrates.
Pockets of decomposing organics are often found in areas of deep fine sand, under rocks or other décor, or in large filters (especially canister filters).
High nitrate levels which many sources based in human studies place as low as 30- 45 ppm of nitrate as harmful. The EPA recommend levels under 10 ppm in drinking water, although often this can higher especially in well water.
As for fish, high nitrate levels can cause respiration problems in fish, lower or eliminate the ability to breed, resist disease, and lower activity of aquarium inhabitants.
However I have kept many general fish from goldfish to cichlids at stable numbers as high as 50 ppm without any noteworthy documented long term problems. I have however noted/documented some long term issues with constant numbers above 50, especially above 100 ppm and higher.
Because of this, as noted earlier, unless your nitrates are showing constant upward trends after every water change, which might indicate a higher bio load than your aquarium system is capable of, do not get too overly concerned with numbers under these.
Further Reading: Bio Load in Aquarium or Pond
Studies show that the toxicity of nitrate is due to nitrite being an intermediate. Nitrites oxidize the iron atoms in hemoglobin from ferrous iron (2+) to ferric iron (3+), rendering it unable to carry oxygen.
This process can lead to generalized lack of oxygen in organ tissue and a dangerous blood condition called methemoglobinemia.
What is noteworthy is that NITRITE also needs to be present, this is why current evidence does not support those in some fish keeping articles that state that anything above 20 ppm nitrate is toxic, as this these assumptions were likely based on nitrite also being present since current scientific evidence shows that nitrates by itself cannot cause these issues at levels at say 30 ppm.
The evidence also suggests that since nitrites were also present, likely such an aquarium was not fully cycled.
In human studies high nitrate levels have been shown to dangerously lower blood pressure by causing the muscles that control the size of blood vessels to relax, this can be dangerous to fish too causing circulatory problems which can again result in poor disease resistance.
High Nitrate levels in aquariums will also result in high algae growths and in marine aquariums is toxic at even low levels to Cephalopods such as Octopus and to corals, although many freshwater fish can tolerate high levels for quite some time based on my experience.
Another danger is in the bloodstream, nitrates can be converted biologically to nitrites, leading to "Brown Blood Disease".
For more on the potential dangers of Nitrates:
*Wikipedia; Nitrate
*Nitrates In Drinking Water
*Wikipedia; Nitrate
Temporary Relief of Nitrate Poisoning:
First, please note that high nitrates are NOT EVEN CLOSE to the danger of high ammonia or nitrites for fish, so if these are the problem, high ammonia and nitrites should be addressed first.
Some have stated that moving from high nitrates to low nitrates or vice versa can also cause nitrate shock similar to pH shock, however this is anecdotal and my tests as well as research have yet to verify such claims (for one nitrates are NOT algorithmic like pH is).
 *Medicated Baths using Methylene Blue can increase oxygen in the blood and quickly remedy ammonia, nitrite, and especially nitrate poisoning (in this order of effectiveness too with ammonia poisoning the least effective and nitrates the most effective).
*Medicated Baths using Methylene Blue can increase oxygen in the blood and quickly remedy ammonia, nitrite, and especially nitrate poisoning (in this order of effectiveness too with ammonia poisoning the least effective and nitrates the most effective).
See Reference: Performing Medicated Fish Baths, Dips, similar
Product Resource: AAP/Kordon Methylene Blu
 *Spirulina Algae or Chlorophyll Remedy; these build the immune system and increase blood oxygen levels in fish that have suffered from nitrite or nitrate poisoning or oxygen deprivation. Spirulina is the better choice of the two, being much better at increasing immune function.
*Spirulina Algae or Chlorophyll Remedy; these build the immune system and increase blood oxygen levels in fish that have suffered from nitrite or nitrate poisoning or oxygen deprivation. Spirulina is the better choice of the two, being much better at increasing immune function.
The most simple and effective way to administer Spirulina is via a high Spirulina based food such as AAP Spirulina 20. Feeding Spirulina based foods although not a replacement for lowering your aquarium or ponds nitrate levels can be a reasonable albeit partial solution to chronic nitrate problems for many fish (not reef inhabitants such as Corals, Cephalopods, etc.).
If you have access to food grade Spirulina or Chlorophyll, these can also be used as a bath:
Pre-mix 1 ounce of spirulina or chlorophyll per gallon of aquarium water (I suggest first liquifying the powder with sterile water to a make a liquid ounce); allow fish to soak for 15-30 minutes; perform this once or twice a daily. Use a fresh bath for each bath using your display tank water.
This can be performed in 1/2 ounces with 1/2 gallon too for smaller fish.
Please read this article for more about Spirulina Algae:
Spirulina Algae
Where to Purchase, Product > *Spirulina 20 Fish Food Flake
*Most importantly, follow the steps below to lower your aquarium or pond nitrates in the first place and often any possible nitrate poisoning issues will go away too.
Here are a few basics for removal/ prevention of nitrates (I will add to this list over time too)
- Water changes; often a simple but effective way to at least temporarily lower nitrates.
Since nitrates often jump considerably after establishing a new aquarium nitrogen cycle or re-establishing the cycle after a problem, a water change is the simple answer, and often no other major nitrate reducing steps needs to be taken.
For high nitrate levels changing as much as 60% then filling the aquarium only 80% (this cuts the nitrates in half), followed by a 50% change again which will then have an over all reduction of 75%.
Also keep in mind to test your tap water as this can affect your nitrate levels as tap (& well) water often have some nitrates in the water already, I have tested as high as 25 ppm (although this is rare).
So as an example you change 50% water that has 100 ppm with tap water that is 20 ppm you will not reduce nitrates by half to 50 ppm.
These water changes assume that your tap or well water is low or lower in nitrates, so make sure to test your "new" water, especially if water changes do not have the impact I described in the previous paragraph.
Of course a constant need to change water for nitrates means you are addressing the symptom, not the underlying problem such as too high a bio load, poor quality food or too much food, and/or poor overall filtration.
With marine aquariums, water changes as a method of nitrate control can be expensive too, not to mention the need to use water changes for nitrate control in marine aquariums might be a good short term method to get a marine aquarium under control, but long term, this indicates an unhealthy marine aquarium (although using a more economical salt mix such as Coralife, Instant Ocean, etc. short term if water changes are being used primarily for temporary nitrate control might lower costs).
Better, after getting nitrates under control in your marine aquarium via water changes is to look into multiple methods of control including deep sand beds, refugiums, protein skimmers, etc. along with the obvious lowering of bio load then utilize a top notch marine salt mix such as Tropic Marin which will long term save money long term when one considers supplements that will not need to be added and longevity of reef inhabitants.
Product Resource: Tropic Marin Salt from AAP - Plants in Freshwater Aquariums (or ponds);
Plants will keep lower nitrate levels. A well maintained planted aquarium maintains lower nitrates by more than one attribute:
- Direct removal of ammonia by some plants such as Hornwort (Foxtail), as well as removal of nitrates from the water column
- The roots also remove nitrates and other nitrogenous wastes.
- Often a well maintained sand, laterite, Plant Grower Bed, etc. around the roots maintain a healthy anaerobic filter bed without reverting to sulfide reduction (Hydrogen Sulfide production), which can often happen in freshwater aquariums where sand is used if no oxygen is allowed to permeate.
- With a pond, nothing works better for nitrate removal from my experience than a Veggie Filter, which when constructed properly includes rooted bog plants along with a good de-nitrifying substrate, generally volcanic rock.
Vivarium, Pothos similar to the Pond Veggie/Bog Filter concept is letting potted plants or a separate chamber with plants above the aquarium be "watered" via aquarium water, this in turn removes nitrates as well as other nutrients from the water column.
Similar is the Refugium concept discussed a bit later in this article commonly used in marine aquariums.
What I find interesting is that many in the hobby act like this concept is some new discovery (generally more common among younger hobbyists), however in an aquarium industry/hobby where mentoring is mostly a thing of the past, they do not realize this concept has been around for decades.
Please Read/Reference:
*Aquarium Plant Care, Information
*Hydrogen Sulfide Production in Anaerobic De-Nitrification for Aquarium/Pond Nitrate Removal
*POND VEGGIE/BOG FILTERS
If plants are being used and you are looking for optimum growth "Aquarium/Pond Answers" recommends the "The Estimative Index of Dosing" (EI) method of plant care which will provide optimum growth with not too much effort if AAP- NilocG Plant Care products are used.
Recommended Resource that supports this frees article: - Vacuuming during most water changes of eventual nitrate producing mulm is essential for both freshwater and saltwater as long as this performed correctly so as to not disturb anaerobic bacteria in saltwater or plant roots in freshwater.
Please see this article: “Aquarium Cleaning” - Lower your bio load/ DOC, as noted in the previous point about vacuuming high amounts of organic mulm/sludge that in turn leads to high DOC (dissolved organic compounds) in the water column is a major contributor of high nitrates. A strong indicator of this problem is a low pH and a KH under 50 ppm along with high nitrates (often over 80 ppm).
Check your filters (especially large capacity filers such as canister or large sump filters) for buildup of mulm/sludge. Under gravel filters can be a major contributors due to trapped decomposing waste under the plates and gravel, especially not "properly cared for" Ornaments, decorative rocks and gravel can also trap copious amounts of decomposing organic waste.
Please also read this article:
Aquarium or Pond Bio Load - Reduce Fish, this includes "Cleaner Fish" or similar; this is similar to the above recommendation as less fish or other inhabitants (crabs, shrimp, snails, etc.) means less organic waste.
As well I should address the often implied Urban Myth about "Cleaner Crews"; adding fish such as Plecostomus to clean your aquarium only cleans cosmetically. The facts are this fish or similar dumps far more organic waste than they take in and in fact while algae might be unsightly, removing it via a plecostomus not only removes a life form that removes nitrates, but adds a life form that adds much more to the nitrates. Snails are even worse in this respect.
I am not suggesting that fish such as Plecostomus should not be kept, but if you are keeping these (along with snails, both freshwater or marine) thinking these are keeping your tank bio load lower and thus nitrates lower, you have them for the wrong reason.
As an analogy; if you had a small child that is constantly spilling and making messes, would you "baby sit" this small child with your dog that is never let out? I think you would find the "Messes" eventually left from the dog to be just as bad and likely much worse. - Reduce feeding and use foods that are more easily digested (made with amino acids that will be used by the fish/aquatic organism and not be expelled) such as Spirulina Algae based foods.
Your choice of food and over feeding can be a MAJOR contributor to DOC and in turn high nitrates, unstable pH, and low KH.
What many do not realize is that excess proteins not only lead to premature kidney (Renal) failure in fish, this also can be a source of high nitrate since nitrogenous wastes start as proteins. YET, many fish foods, that many unfamiliar with long term fish nutrition research think are top notch do not properly limit proteins and also still have to supplement otherwise natural ingredients resulting in a less than optimum fish food. Examples of popular and otherwise good fish foods that do NOT properly limit and utilize proteins include Repashy & NLS. Better is an all natural fish food that is designed with the history of fish food research behind it that has optimum protein levels and ingredients that work best (not just are popular). First Paradigm met this, but later the creator of the formula improved the formula and now even better limits protein and it is now offered as AAP Custom Ultra Premium Fish Foods.
Better is an all natural fish food that is designed with the history of fish food research behind it that has optimum protein levels and ingredients that work best (not just are popular). First Paradigm met this, but later the creator of the formula improved the formula and now even better limits protein and it is now offered as AAP Custom Ultra Premium Fish Foods.
Product Resource: "AAP Premium All Natural" Custom Fish Food Crumbles - In marine aquariums the use of "Live Rock", Mud Filters/Refugiums, and/or Deep Sand Filters can help reduce nitrates.
Mud or Deep Sand Filters and Refugiums remove nitrates while Protein Skimmers remove nitrogenous wastes (proteins) before they enter the nitrogen cycle. .
I prefer the more natural use of live rock (as well as live rock crumbles in filters) and mud filters/Refugiums as well as Mangrove Plants and Caulerpa algae in my marine aquariums. Good deep sand (#00) beds of over 3” (with a ½” layer of #3 crushed coral on top for ease of cleaning) also are helpful as these allow anaerobic bacteria to form and remove nitrates.
Further Reference:
Marine Aquarium Care, & Basics Large Live Rocks which have now been popular in marine aquariums since the 1980s as decorations that also act as bio filters, are both a positive AND a negative, depending upon use.
Large Live Rocks which have now been popular in marine aquariums since the 1980s as decorations that also act as bio filters, are both a positive AND a negative, depending upon use.Let me explain:
- An aged (seasoned) Live Rock specimen will have aerobic bacteria in the outer calciferous surfaces that remove ammonia and nitrites and anaerobic bacteria on the inner calciferous surfaces that remove nitrates.
- However often owners of new, unestablished saltwater aquariums will purchase Live Rock from a retailer that is NOT seasoned.
The results is this live rock is not so "live" and in fact has a lot of decaying material within the calciferous surfaces of the rock that instead raise ammonia, nitrites, and nitrates.
If your marine tank is well established and you add this unseasoned live rock (in increments), likely there will be no problem.
But if your tank is under 12 weeks of ages, I would urge the reader to inquire as to how long the retailer has had the so-called "live" rock; if under 8 weeks, do NOT purchase.

Another method for marine aquarium nitrate control is a simple deep sand bucket utilizing #00 oolitic sand.
The picture to the left demonstrates a simple DIY method, please click on the picture to go to the website describing this in more detail and for a larger image. - Use of Reverse Osmosis (RO) or RO/DI Water
PLEASE READ this article for a much better understanding of what to look for and how to use a RO or RO/DI system/filter:Use of RO or RO/DI water in part or in full, ASSUMING correct re-mineralization can reduce or totally remove nitrates that are added via a water change from tap or well water.
Be aware that a premium well constructed RO system such as the AAP/TMC is all that is generally needed as these will utilize better micron pre-filtration, catalytic carbon, optimum construction that maintains water pressure and does not allow flow by, and most importantly a medical grade TFC RO membrane.
Do not be misled by popular bulk online sellers that are selling large multistage RO in combination DI systems as the before mentioned RO only system will remove 97% of all nitrates without the cost of DI chamber resins on a regular basis.
This slick marketing has misled many in the hobby even though the need for a DI chamber for nitrate removal when using a well made RO ONLY system was discredited YEARS AGO. The result of is the need to re-purchase DI resins thus fulfilling the quote misattributed to PT Barnum: "There’s A Sucker Born Every Minute"
So unless you are making water that needs to be 100% pure of ions (such as topping off deep cycle RV/Marine batteries) or if you have purchased an inferior RO system, there is no need of a DI system for nitrate removal!!!
Use of RO, DI, Softwater in Aquariums
Product Resource: AAP V²Pure Premium Reverse Osmosis Water Filter System - The use of a Protein Skimmer.
 In marine aquariums is an effective way (depending on the skimmer) of removing ‘protein’ based organics BEFORE they can enter the nitrogen cycle and thus become nitrates.
In marine aquariums is an effective way (depending on the skimmer) of removing ‘protein’ based organics BEFORE they can enter the nitrogen cycle and thus become nitrates.
These devices generally do not work in freshwater as they work via foam refraction which is a process that generally does not work well in freshwater.
As well, there is also evidence that foam refraction will be limited by the use of carbon since carbon removes MBAS Foaming Agents.
See this article for more: Activated Carbon, Uses, Adsorption, Absorption.
For Reef tanks, these devices are almost a must, and generally the low end skimmers are not adequate, high end skimmers such as Warner Marine mesh wheel skimmer or the Tropic Marine V2 Skimmer are among two of many good choices.
I personally feel some older style protein skimmers can be over rated due to the often erratic upkeep they require (although the newer models are often worth the expense in both effectiveness and lack of hassles).
Also be careful of many downdraft/spray injection Skimmers that do not have the vertical reaction ability (example the "Remora") Adding an Ozonizer will often significantly improve efficiency by converting fish waste into more easily removable by-products. If nitrates are a problem even with a protein skimmer (especially with with smaller skimmers), this IS A MUST HAVE DEVICE!
Adding an Ozonizer will often significantly improve efficiency by converting fish waste into more easily removable by-products. If nitrates are a problem even with a protein skimmer (especially with with smaller skimmers), this IS A MUST HAVE DEVICE!
These work most simply with a Venturi style skimmer, but adding an air pump to a needle wheel skimmer will also allow use of an Ozonizer/Ozone Generator.
Please see this article for more information about Protein Skimmers:
Marine Aquarium Protein Skimmers; Review - Use of AAP Bioplastics Nitrate & Phosphate Reducing Polymer Media
 This is a if not THE top notch method for BOTH effective nitrate reduction as well as phosphate reduction (both of which when combined can be very detrimental to marine aquarium hard corals).
This is a if not THE top notch method for BOTH effective nitrate reduction as well as phosphate reduction (both of which when combined can be very detrimental to marine aquarium hard corals).This product was originally intended primarily for marine aquarium use and it also requires a protein skimmer to be in use for best results.
However use in a freshwater aquarium has been shown to also be VERY effective, if not the most effective method for problem nitrate levels and high bio loads. Some of the bacterial by-products produced by AAP Bio-Plastics may be assimilated into the plant root structures in planted aquariums, as well the use of SeaChem Purigen may help further remove by products that may yellow the water.Normally the use of Purigen in planted aquariums is not suggested for long term use, but with AAP Bioplastics, temporary use to clear yellow water would be an exception.
With a fish only freshwater aquarium, use Purigen long term as well as use of Wonder Shells or Wonder shell fragments can offset any Redox Balance issues.NOTE: Make sure your FSB filter or similar reactor is properly tumbling the Bioplastics. This can be a very effective and simple to use product, but not used properly in the proper device, the conditions can become anoxic and allow for hydrogen sulfide production.
Running with a pump or filter that powers the FSB filter or reactor to the minimum line is important, but as well I suggest the use with silica or oolitic sand too as would be the case in a good FSB filter (such as the TMC models) since this sand breaks up the Bioplastics. This is why the best device to use for this product is a FSB filter over a reactor.
Below is picture that displays the results when used in a planted freshwater for just one week:While this product can be placed in any high flow filter, it is best utilized in a reactor or best in a Fluidized Filter

Here is just one reference from a user of this VERY effective nitrate reducing product:
www.aquariumforum.com/f20/bioplastics-38846.html
Where to purchase, product references:
*AAP Bioplastics Nitrate & Phosphate Reducing Polymer Media, By Two Little Fishies
*AAP Fluidized Aquarium Filters
*SeaChem Purigen; Removes Water Yellowing Compounds - "Vodka Method"
As with the above method, this is only for marine use and requires a Protein Skimmer. This method is also less exact and simple along more issues with Gelbstoff which is a yellowing of the water and slime as a side effect, so the use of an Ozone Generator is also strongly suggested.
That said, it is still an effective method for both nitrates and phosphates in marine reef aquariums.
See this excellent article for further information about this method:
Vodka Dosing  Sulfur Denitrators: These are growing in popularity, in particular in Europe. My experience with these is limited, nor has too much positive feedback from those I trust in the professional aquarium maintenance business reached me with these, but that does not mean these cannot work either.
Sulfur Denitrators: These are growing in popularity, in particular in Europe. My experience with these is limited, nor has too much positive feedback from those I trust in the professional aquarium maintenance business reached me with these, but that does not mean these cannot work either.Sulfur De-nitrators rely on anaerobic bacteria, colonizing a suitable medium (such as the CaribSea LSM Live Sulfur Media) and the flow of water being slow enough, to become depleted of oxygen to create an environment these bacteria can thrive in. These bacteria will then multiply and consume the nitrate in the low oxygen water.
Please note that many still consider this as an experimental process.
A typical Sulfur De-nitrator system employs two separate stages or canisters with a means to control water flow rate through the reactor. The first canister contains the sulfur reducing medium. The second canister is filled with aragonite to neutralize acidified water and supply calcium ions as a by-product of neutralization. LSM™ can be placed in any oxygen restricted environment.
Nitrate and pH test kits are required to fine tune any nitrate reducing equipment. Sulfur based nitrate reducing bacteria require an oxygen depleted environment.
Be aware that this process may strongly acidify surrounding water.
Please further note, that nitrate reduction with sulfur may produce an odor!- High Porosity Bio Media: I have had success in preventing this problem with the use of live rock crumbles in place of much of the standard bio filter media in saltwater aquariums so as to allow for de-nitrification deep inside these crumbles. Volcanic rock is a substitute that works well too for this and can be used in FW as well.
As to volcanic rock and live rock crumbles, the higher the flow rate, the larger the volcanic rock/crumble size so as to allow for correct anaerobic de-nitrification (generally about a ¾ to 1 inch diameter rock size for flow rates less than 350 gph).
Product Resource: Volcanic Rock Aquarium and Pond Filter Media
Eheim (Substrat Pro) and JBL (MicroMec) are similar products (in both cases, sintered glass) and are claiming larger specific surface areas than for Matrix, however there is a second consideration, and that is the size of the pores in the medium. Another product I have used with good results, probably the best of these bio nitrate removing mediums, is SeaChem’s Matrix de-nitrifying bio media.
Another product I have used with good results, probably the best of these bio nitrate removing mediums, is SeaChem’s Matrix de-nitrifying bio media.
Generally, with very large pore diameters, we have smaller specific surface area, so that is not good. This generally rules out pores above 10 microns in diameter.
But we can go too far in the other direction as many products such as Eheim (Substrat Pro) and JBL (MicroMec) have done.
If we have a very large number of very, very small pores, then our specific surface area number will be phenomenal, but the medium will not work very well as a biological medium. This is due to physical limitations, specifically too small a volume to support bacterial growth, and the decreasing efficiency of fluid transport (necessary to carry nutrients to the bacteria and waste away from the bacteria) with very small pore sizes.
Regardless of which of the above de-nitrifying bio media you choose, these do NOT remove nitrates over night unlike chemical filter media such as Purigen, rather over time nitrate removing anaerobic bacteria form in these medias that slowly convert nitrates to free nitrogen (thus expelling the nitrogen to the atmosphere).
Generally you need to allow 4 weeks plus to see measurable results, but once the results start these de-nitrifying bio media rarely need to be changed.
With canister filter use, these high Porosity Bio Media are best placed near the beginning of where water enters your filter (first or at least 2nd stage), unlike Purigen which is best used near the final stage.
These bio media are safe and often best combined with other methods such as Purigen, AAP Bioplastics, etc.
Where to purchase: SeaChem Matrix; High Porosity Bio Media
*Please note that many otherwise excellent bio media such as bio balls, bio stars, ceramic bio rings, etc., only perform nitrification, that is the conversion of ammonia and nitrites, but this only adds to nitrates as these products do NOT have the pore size and too much oxygen is available to the pores that are found on these products which oxygen in any quantity inhibits de-nitrification (the removal of nitrates). - Macro-Porous Synthetic polymers, Ion Exchange Resins, Absorbents, etc.; there are several nitrate “sponge” or resins available, although these can be costly. Zeolite can be used in freshwater aquariums to remove ammonia before it ever goes thru the nitrogen cycle, eventually becoming nitrates.
API Bio-Chem Zorb Chemical Filter Media is an good example of a blended carbon and resins that can aid in nitrate control.
This is an excellent product for those who need more than just carbon, but do not have a serious nitrate problem since this product and all similar are best just for control of nitrates, not serious issues (use with other control products such as Algone can increase effectiveness when nitrates are a more moderate or serious issue)
 Another good product is SeaChem’s Purigen which more effective for more serious nitrate problems than BioChem Zorb (although it does not contain carbon).
Another good product is SeaChem’s Purigen which more effective for more serious nitrate problems than BioChem Zorb (although it does not contain carbon).
Purigen be used in both freshwater and saltwater and is specifically designed to be an organic scavenging resin.
Purigen generally ignores simple elemental compounds, having an extreme affinity for nitrogenous organics which include the cause of yellow water for which this product excels in "water polishing" as well as some nitrate removal.
However, Purigen along with other "Synthetic polymers, Ion Exchange Resins, Absorbents" cannot remove the volume of nitrates many/most aquariums require, so this product is best used with others especially AAP BioPlastics (Bioplastics must be used in a reaction chamber such as Fluidized Sand Bed Filter). Please read the earlier section about Bioplastics.
Purigen can also be regenerated with bleach, however do not use in combination with Stress Coat.
For more about this please read this article: Aquarium Answers: Aquarium Water Conditioners .
Purigen can also raise your Redox, so maintain a Redox balance with the use of additional minerals such as Wonder Shells.
Reference: Aquarium Redox, Balance, Potential, Reduction, Oxidation
Where to purchase, product resources:
*SeaChem Purigen Premium Synthetic Adsorbent
*Wonder Shells-Medium; Improves Mineral Cations, Redox Balance - Organic Nitrate Reducing Products; such as AAP Algone for freshwater or saltwater can aid in nitrate prevention/removal.
 In fact in our aquarium maintenance companies controlled tests we found differences in aquariums (both freshwater and saltwater) in Nitrate levels between tanks with and without Algone Nitrate Controller when used as a nitrate control maintenance product.
In fact in our aquarium maintenance companies controlled tests we found differences in aquariums (both freshwater and saltwater) in Nitrate levels between tanks with and without Algone Nitrate Controller when used as a nitrate control maintenance product.
Algone works by utilizing Nitrate fixating microorganisms which incorporate excess nitrogen into the cellular mass, while bio active enzymes assimilate nitrogen from the water column.The concept (not the process) is similar to the use of a Marine Protein Skimmer, in that many nitrogenous wastes are removed without going through the nitrogen cycle. For this reason, I do not recommend using Algone in new tanks, only established fully cycled aquariums (as I would a Protein Skimmer).
My personal recommendation for Algone use is to use it as a nitrate control maintenance product as alluded to earlier. This means that for aquariums with average bio loads that simply keeping nitrate numbers in check, this product is excellent for.
However in high bio load aquariums, this product should be more used as part of a nitrate lowering problem or as a nitrate controller once lowered by other means (including water changes) as often Algone is not quite enough in these scenarios (AAP Bio-Plastics is the better choice or should be used along side Algone)For those who have tried this product with only poor results, often it has been used in correctly.
Where to purchase: AAP Algone for Nitrate Control, Water Clarifier in Aquarium
Our large aquarium maintenance company has used AAP Algone extensively and has found best results by using it more as a product to control nitrate once lowered and just as importantly placing the AAP Algone in a low flow area such as at the base of a lift tube or sponge filter or in a sectioned off section of a high flow filter so as to keep the flow slow/low around the Algone. - Use of an Algae Scrubber:
This can be a traditional algae scrubber that is often part of a marine aquarium Refugium as demonstrated in a picture found in this article:
Marine Aquarium Set Up Suggestions
Picture Link: Algae Scrubber/ Refugium Picture
See the picture to the left of a HOG1.3 that attaches magnetically to be sold by American Aquarium Products
As a new alternative that works with both freshwater and saltwater aquariums are the Hang on Glass Up flow with LED grow light algae scrubbers.
These grow algae inside the unit that lower nitrate and phosphate by removing these nutrients from the water column. In turn this can control many nuisance algae and create a better environment for plant growth and coral growth.
Here is a YouTube Video for a DIY algae scrubber
HOW TO: DIY algae scrubber - No more nitrates
Please note, I would recommend the use of a T2 light over a desk lamp for higher efficiency growth
- Use a re-circulating micron cleaning filter so as to remove excess mulm/detritus from your aquarium without over cleaning (meaning excessive water changes over 50%) or in between regular water changes.
The key is this device (& similar devices such as the Vortex Diatom filter) efficiently removes fine organic particulates BEFORE they would otherwise go through the nitrogen cycle. The Eheim Detritus Extractor Battery Gravel Vacuum is a useful device for this purpose as it removes many of the organics before they are converted via the nitrogen cycle to nitrates (similar in theory to a protein skimmer, albeit a very different process).
The Eheim Detritus Extractor Battery Gravel Vacuum is a useful device for this purpose as it removes many of the organics before they are converted via the nitrogen cycle to nitrates (similar in theory to a protein skimmer, albeit a very different process).
The use of this or similar recirculating filters/vacuums prevents the formation of nitrifying bacteria that would break down organics resulting in higher nitrates. The theory is similar as to how a protein skimmer works in marine aquariums, however this machine and similar can be used in salt OR freshwater!
Where to purchase: *Eheim Sludge, Detritus, Mulm Extractor Battery Gravel Vacuum - Rinse filters often with de-chlorinated water to prevent organics/mulm build up. This is especially important in “nitrate factory” filters such as Wet-Dry and canister filters but can apply to ANY aerobic bio-filter.
Even with high efficiency sponge filters this is important, especially in high bio load environments.
I have heard some aquarium keepers state that they have gone months without rinsing with no discernible increase in nitrates. I do not doubt this as per my controlled tests and observations, HOWEVER in more heavily loaded aquarium environments my tests/observations HAVE SHOWN lower nitrates by increasing frequency of rinsing ANY aerobic filter medium.
How this works is that by more frequent rinsings, you are removing organics trapped in the filter media before it even is broken down via the nitrogen cycle.
This is probably most noteworthy with canister filters and Wet/Dry filters. These filters have the reputation within the saltwater side of the aquarium keeping hobby in particular as “nitrate factories” due to the fact that these filters both trap a lot of organic debris and also are very efficient aerobic nitrifying filters yet do little for de-nitrification.
Rinsing bio media is helpful for these filters as well as increasing frequency of this procedure; this also applies to freshwater as well.
Adding a good pre-filter such as the AAP Filter Max is a good way to lower the Canister Filter "Nitrate Factory" effect. While a good sponge pre-filter also produces nitrates, these are extremely easy to rinse, even daily if need be, to lower the nitrates being otherwise produced by a canister filter.
Beware that using just a "replacement" sponge from an AAP Filter Max on the strainer does not work the same as the complete pre-filter and in doing this to save a few dollars results in only about 25% of the effectiveness of the complete Filter Max.
Product Resource: AAP Filter Max #3
In heavily planted freshwater aquariums, frequent rinsing is often not necessary as the plants will often consume most extra nitrates and over cleaning can actually reduce nitrates too low for heavily planted tanks with CO2. However in marine aquariums this IS essential.
Resource for Sponge Filters:
THE PREMIUM AAP Aquarium Sponge Filter with as much as 5 TIMES the bio and mechanical capacity of commonly sold Chinese knock offs!!
Definitely worth the extra $1-3 - The use of fine micron poly filter pads inside easy to access filters such as aquarium power filters (HOB) or wet/dry filters.
Where to purchase Mineral Cation replenisher:
I would place these in easy to reach spots and NOT use them to replace the regular filters or sponges, rather place a piece in front of the existing filter.
This pad needs to be rinsed every 1-3 days. How this works is this filter material will trap fine organic particles BEFORE going through the nitrogen cycle which would otherwise result in nitrates.
The key is regular rinsing in CHLORINATED water so as to prevent the formation of nitrifying bacteria that would break down organics resulting in higher nitrates. This is similar to how the micron cartridges work (except on a much smaller and less efficient scale) in cleaning filters.
I recommend generic versions rather than the more pricey name brands which can be cut to fit
Product Resource: Generic Fine Micron Poly Filter Pads
The use of or micron socks, often employed in marine reef systems can achieve similar results and should be rinsed often as well for best results.
Another popular product is the "The Poly Pad". The claim is these also remove heavy metals such as copper as well as phosphates, ammonia, and nitrates as well as certain organic via proprietary matrix of synthetic poly fibers.
HOWEVER despite some claims by popular aquarium YouTube channels that have not done their homework, these are not as effective as the claims based on tests I have performed. MORE IMPORTANTLY; these can severely affect Redox balance resulting in pH crashes and an unhealthy oxidizing environment that used long term can be harmful to Fish immunity.
I am not saying these cannot be safely used, but care should be exercised along with the use of Mineral Cation replenishers (& water changes) to neutralize the negative affects of this product.
Further Reading:
Aquarium & Pond Filter Media, Material; Mechanical, Bio, Chemical
*AAP Wonder Shell Large, Mineral Cation Replenisher - Check your clean water source as I have tested Tap Water in excess of 20 ppm and some wells may also be high. Please note that the USA EPA has set maximum of 10 mg/L (or 10 parts per million) for the safety of drinking water.
The use of “clean water” that already has moderate amounts of nitrates lessens the effectiveness of water changes. For SW the solution is simple, I recommend the use of Reverse Osmosis, DI (Distilled) or similar water for mixing all new saltwater and for topping off for evaporation.
For FW, this is a bit more difficult as RO/DI water is missing VERY essential elements for fish osmoregulation so its use is not generally acceptable (the use of marine salt mix replenishes ALL necessary elements back to the water). At best the FW aquarist can uses partial RO, DI or similar and then reconstitute the water with minerals with products such as Wonder Shells, Alkaline Buffers, and Cichlid Salts.
Recommended Further Reading:
Tap Water, Use in Fresh and Saltwater Aquariums
Where to purchase, product resources:
*TMC RO Aquarium Water Filter Systems 50 gpd
*SeaChem Marine, Alkaline for Fresh and Saltwater
*SeaChem Cichlid Salt, Minerals for most Freshwater Fish - Carbon does not remove nitrates; although the use of carbon has its supporters and detractors, one aspect of activated carbon that is clear is that it cannot remove nitrates. Activated carbon filtration does not remove microbes, sodium, nitrates, fluoride, and hardness. HOWEVER Carbon can remove DOC, which eventually can lead to high nitrates, so the use of carbon in aquariums with nitrate problems is certainly a worthwhile endeavor.
Please reference the more in depth Aquarium Answers article about the use of carbon:
Aquarium Answers; Activated Carbon
Further Resources:
Please reference this other Aquarium answers article for more other filter media types as well (including products for phosphate removal), a MUST READ:
Aquarium Filter Media; Types, Capacities and more
Please reference this article for more about the production of Hydrogen Sulfides during the process of De-Nitrification:
Hydrogen Sulfide production in anaerobic De-Nitrification for Aquarium/Pond Nitrate Removal
For a map of Nitrates in World Rivers, please click below
Nitrate Levels in Major World Rivers
For more referenced aquarium information pertaining to:
*Freshwater Aquarium Care, Basics to Advanced
*Saltwater Aquarium Care, Basics to Advanced
Other Recommended Reference & Product Sites/Videos

One of the most effective medications for the treatment of Columnaris in an aquarium when used as part of the four step program of Columnaris treatment.
A more synergistic combination than purchasing Kanamycin & Nitrofurazone separately.
OR
AAP Yellow Powder; The Premium Nitrofuarazone combination; for Columnaris and Aeromonas next to Spectrogram (as Spectrogram is often not available), the best treatment for Columnaris, despite bad search results recommending Terramycin (oxytetracycline) and other inferior treatments for Columnaris

YouTube; How to: 4 Steps Columnaris Treatment Fish Bacterial Infection
This video goes over the basics of the full four step plan of properly treating Columnaris in aquarium fish and is a compliment to a FULL reading of this article.
The article below is a MUST READ for anyone interested in moving from basic aquarium keeping to more advanced aquarium keeping, including better Redox Balance:

Ultraviolet Sterilization, Advanced Aquarium Keeping

POND CARE INFORMATION; Complete Steps

Premium Tropic Marin Pro Reef Sea Salt from Germany
There is simply NO BETTER Reef Sea Salt (marine fish too)
 UV Replacement Lamps/Bulbs; Aquarium or Pond
UV Replacement Lamps/Bulbs; Aquarium or Pond
For TRUE High Output, Hot Cathode, Low Pressure UVC Germicidal Bulbs, for aquarium or pond
*Sponge Filtration; Complete Information about the use of Sponge Filters in Aquarium or Pond
 FISH AS PETS
FISH AS PETS
Fish as Pets with articles & commentary of Interest to the Aquarium Hobby
 SunSun JP-023 Aquarium Power Head Water Pumps
SunSun JP-023 Aquarium Power Head Water Pumps
Superior performance and value when compared to many more well known brands such as Hagen or Marineland
ADVERTISEMENT
Labels: aquarium, aquarium nitrates, aquarium nitrogen cycle, Aquarium Pond Answers Directory, Aquarium Test Kit ammonia multi strips titration GH KH pH, cleaner crew, Fish, nitrates, nitrites, pond, pond nitrates