Our Facebook Page to Follow: Aquarium/Pond Answers Facebook
This is a unique resource for answers, help, & advice to aquarium and pond questions not found elsewhere; With regular posts & article updates.
In our research; we use aquaculture, horticulture, medical, & university research to compile many of our articles.Our Recommended Lighting for highest efficiency professional planted/reef aquariums: "AquaRay Lighting"
Salt Use (Sodium Chloride) In Freshwater Aquariums
By Carl Strohmeyer-PAMR 40+ years experience
Aquarium Salt (Sodium chloride) in Freshwater Aquariums
Updated 10/24/20
 The use of Sodium Chloride more commonly known as plain salt seems to be a constant source of controversy among aquarists, especially here on the internet. What is interesting to me and my colleagues in the professional aquarium maintenance, design and research business is that the way this controversy has “swung” from "you MUST use salt" to cure everything to the now current fad propagated by many forums and articles, to "RARELY or NEVER use salt".
The use of Sodium Chloride more commonly known as plain salt seems to be a constant source of controversy among aquarists, especially here on the internet. What is interesting to me and my colleagues in the professional aquarium maintenance, design and research business is that the way this controversy has “swung” from "you MUST use salt" to cure everything to the now current fad propagated by many forums and articles, to "RARELY or NEVER use salt".
Unfortunately, both views are based on misunderstandings of the term of what salt is and what fish need “salts” for, as well as a lack of understanding and reading of research about this subject.
Please read the article in full, including the myths/truths section.
What is “Salt”
I will start with some basic definitions of what “salts” are. Further reading beyond this article is needed here, so please follow links/resources/references.
Basically, a salt is a neutral compound composed of cations (positively charged ions) bound to anions (negatively charged ions).
A more in depth description is that “salts” are ionic compounds held together by electrostatic attraction of positively charged metal cations and the negatively charged anions. These Ions can be simple molecules, as in sodium chloride, or more complex groups such as calcium carbonate.
What I am driving at is that salts consist of more than just Sodium Chloride (what we generally refer to as salt is sodium chloride), and salts in general are IMPORTANT electrolytes necessary for biochemistry and osmoregulation in fish. Without salts fish and other biochemical processes would cease.
Please reference these two articles for further reader as to this subject:
*PROPER OSMOTIC FUNCTION- ELECTROLYTES; DO FISH DRINK
*AQUARIUM CHEMISTRY; How to maintain a Proper KH & PH, why calcium and electrolytes are important.
Common salt-forming cations applicable to aquarium keeping include:
- Ammonium NH4+
- Calcium Ca2+
- Magnesium Mg2+
- Potassium K+
- Sodium Na+
Common salt-forming anions applicable to aquarium keeping include:
- Carbonate CO32- (carbonic acid)
- Chloride Cl- (hydrochloric acid)
- Nitrate NO3- (nitric acid)
- Nitrite NO2- (nitrous acid)
- Phosphate PO43- (phosphoric acid)
Referenced/Sources from: What are Salts; Wikipedia
ADVERTISEMENT
Sodium Chloride and other Salts in Aquariums
 This where the controversy begins in my opinion. The question is, should aquarium salt (sodium chloride) be used and if not, what will take its place for necessary electrolytes?
This where the controversy begins in my opinion. The question is, should aquarium salt (sodium chloride) be used and if not, what will take its place for necessary electrolytes?
First, I will start off by stating again that ALL living organisms need certain electrolytes for biochemistry. The before mentioned resources help explain this fact).
I have read many scientific studies as well as performed many experiments over the years in the use of different salts including sodium chloride.
I will start by stating that a successful aquarium CAN be kept without sodium chloride HOWEVER, other "salts" MUST be present such as Calcium carbonate, otherwise your fish will have lower disease resistance and other physiological problems.
As a generalization when it comes to sodium chloride salts, I have found 1 tablespoon per 5 gallons works safely as a preventative for many community aquariums. However in most community aquariums, I have found in experiments in multiple aquariums, keeping almost no salt but then adding salt at a tablespoon per 5 gallons or higher when any possible issues arise or new fish are added works best (keeping in mind salt is not a cure all).
For therapeutic levels in aquariums or baths, 1 Teaspoon per one gallon is a good starting point (it can be higher in many cases).
Where this gets controversial is with freshwater Plants, Catfish, Elephant Nose, Tetras, Goldfish and Livebearers such as Mollies.
Starting with each of the above, let’s take a look them specifically:
- Freshwater Plants;
As many articles state correctly, many freshwater plants (not all though) have a low tolerance for sodium chloride, so care must be exercised in the use of sodium chloride (NaCl) with many plants present.
Most freshwater plants can tolerate up to 1000 mg. per liter of sodium chloride and since a teaspoon is roughly 5500 mg. that equals one teaspoon per 5.5 liters of water or 1.45 gallons (one gallon = 3.785 liters). This is approximately 1 Tablespoon per 5 gallons.
Please keep in mind that this is the upper reach of many plants tolerance, so a lower amount would be better.
This said, I generally have used very little sodium chloride salts in my planted aquariums (Anubias one of the exceptions), however the plants still require other electrolytes, (salts) so one must make sure that calcium carbonate, magnesium and other necessary minerals ("salts") are present.
Much more about: Freshwater Plant Care - Catfish, Elephant Nose, etc;
This is an area where my own research as well as university level research does not “mesh” with current popular opinions/fads.
While it is true that Catfish, Tetras, and fish such as Elephant Nose do not tolerate salt well, they still MUST have some electrolytes and can tolerate some salt, at least in short term doses.
Fish such as Elephant Nose & Knife Fish that navigate by electrical field do not do well in higher doses of salt (NaCl), these fish can tolerate short term doses of salt such as after introduction of new fish for aid in disease resistance, transport or aid in disease treatment.
Generally, long term sodium chloride use should be kept to levels under 500 mg. per liter or less, I recommend no more than 1 teaspoon per 5 gallons for these fish. This is one TEASPOON, not Tablespoon!
Since there is little money for really good studies for most aspects of aquarium keeping I, and others serious about really good research, must look to outside sources for information. This includes other areas such as Aquarium Lighting and other poorly researched subjects within the aquarium industry.
Where I am going with this point, is that outside of my own tests and side by side studies (admittedly not to university level standards) most good research as to salt (NaCl) comes from studies in food grade fish such as Channel Catfish.
Example of Researched Aquarium Lighting Information:
Aquarium Lighting
These studies are not 100% to extrapolate results for since Channel catfish are not Corydoras Catfish, however based on my own studies as well, these studies are still VERY useful for a scientific understand of the use of sodium chloride with fish such as Corydoras Catfish.
The most current studies I have read show that Channel Catfish CAN tolerate salt in surprisingly high amounts for at least short periods of time with no long term issues.
In fact studies have shown that treatment of Channel Catfish for Columnaris when sodium chloride is used vastly INCREASED the survival rate! These studies showed increased survival in concentrations between 1000 and 3000 mg. per liter.
This comes out to .67 teaspoons per liter or 2.54 teaspoons per gallon!
Reference: Alabama Agricultural Experimental Station, Auburn University
This would also include my own experiments which consisted of literally 1000s of tests/applications over three decades with direct aquarium application and more so with fish baths and fish dips where salt (sodium chloride) is utilized as one of the ingredients for the bath or dip.
Bringing this back to aquarium keeping and my own studies/results as per catfish, I found no problems in short term use up to 2 tablespoons per 5 gallons and long term use up to one teaspoon per 5 gallons.
Please also see this article for a graph from a University Study of salt use for Channel Catfish:
Columnaris/Saprolegnia Treatment, Prevention, Identification - Tetras;
As with Catfish, most Tetras originally inhabit waters that have little or no Sodium Chloride, however as with ALL fish Tetras must have at least some minerals/electrolytes per. For short term use (new fish, disease control, stress, etc.), 1 tablespoon per 5 gallons or even 1 teaspoon per gallon can be easily used with most Tetras.
For long term use I generally recommend/use no more than 1 Tablespoon per 5 gallons or no salt at all, HOWEVER I still keep minerals/electrolytes in Aquariums with Tetras at a level of 100 ppm GH or higher (Wonder Shells can be used as an aid for correct mineral levels).
I generally keep carbonates/bicarbonates (as with catfish) at around 50-100 ppm KH for stability of pH, but not so high as to maintain high pH of more than 7.5 (although pH stability is what is most important and actual pH is only a minor secondary concern.
The addition of Indian Almond Leaf power, extract, or similar products are helpful in keeping healthy Tetra Aquariums by adding beneficial natural tannins (& as well control Aeromonas Bacteria), as found in products such Pillow Moss.
PLEASE see this article in the Amazon River section for more about keeping a tank that would be excellent for many Tetras: Aquarium Chemistry; Amazon River Water
Product References:
Wonder Shells
Pillow (Frog) Moss; Natural Water Softener - Goldfish;
Gold fish are from a family of fish that include koi and carp. The common goldfish (Carassius auratus) and its fancy variations are fish based on my own experience that do better in water that contains moderate amounts of minerals and electrolytes.
This includes a GH (for Calcium, magnesium and more) as well as some sodium chloride salt.
My clients goldfish have always had more longevity and less incidence of disease when the GH is 200 ppm plus (Wonder Shells are one way for maintaining correct mineral levels) and I have kept 1 tablespoon of salt per 5 gallons of water.
In fact a government study shows INCREASED survival rates when chronic salinity levels were increased up to 6000 mg. per liter (6 ppm).
I will also note as per goldfish that these are a fish that I have performed several tests/studies as per the use of salt, use of UV Sterilization and improved Aquarium Redox.
These facts fly in the face of the many anecdotal postings of what is best for healthy goldfish.
Further References:
How UV Sterilization works
Benefits of a proper Aquarium Redox Potential - Livebearers such as Mollies;
This is another area where I find some miss-information. Most aquarists would agree that mollies will do fine with sodium chloride salt in their aquariums, however what many aquarists miss is that mollies do not all come from areas with NaCl in their water but ALL mollies and livebearers in general do and must have other electrolytes such as Calcium Carbonate and Magnesium in their water as well as buffers such as sodium bi-carbonate.
Personally I have had the best results with my livebearers with a GH well over 200 ppm (for calcium/magnesium), a KH over 150 ppm and finally 1 tablespoon of sodium chloride per 5 gallons.
If salt is kept with livebearers, I often use marine salt and then I will use the best possible to provide natural bio available salts & pharmaceutical ingredients to insure high purity.
For this I recommend Tropic Marin Reef Salt from Germany (which is sold by the pound to make it more readily available for smaller uses).
Product Resource: AAP-Tropic Marine Pro Reef Salt from Germany
Please see this article for more: “Keeping Molly Fish in Aquariums” - Cichlids;; in particular African Cichlids are commonly kept with some salt in the water.
My personal tests as well as research has shown that although African Cichlids need high amounts of minerals (high GH and KH), they do not necessarily need salt (sodium chloride).
I have found better results keeping Mbuna, Haps and other Rift Lake Cichlids to maintain high levels of minerals in particular calcium and carbonates (via a GH of 200 plus and a KH of 150 plus), and then reserve the use of salt (sodium chloride) for times of stress, new fish, or disease such as Ich or Columnaris.
I have found better results with this restrained occasional use of salt while I regularly use products such as Wonder Shells for minerals.
The Cichlids then respond to treatment better when salt is only added during disease treatment, when new fish are present, or other forms of stress. As well, the incidence of Malawi Bloat is reduced since over use of salt is a contributing factor in some causes of Malawi Bloat. - Brackish Fish;
Brackish water infers bodies of water that has more salinity than fresh water, but not as much as seawater.
Technically, brackish water contains between 0.5 and 30 grams of salt per liter, often expressed as 0.5 to 30 parts per thousand, which is a specific gravity of between 1.005 and 1.010.
Some brackish fish include before mentioned livebearers such as guppies and mollies, but as noted NOT ALL guppies or mollies hail from areas of elevated salt (sodium chloride).
HOWEVER more traditional brackish fish that should ALWAYS be in water with some sodium chloride include: Green Spotted, & Figure 8 Puffers, Monos/Moonies (Monodactylus argenteus, & Monodactylus sebae), Bumblebee Gobies, Archer Fish, Siamese Tigerfish (Datnioides microlepis), and American Flagfish.
True brackish fish should always be kept in water containing QUALITY marine salt at a specific gravity of between 1.005 and 1.010. The use of plain aquarium salt or even cheaper marine salts that are commonly sold will often result in failure or less healthy brackish fish.
The reason in part is because these fish live in water of lower salt concentrations of mineral salts (& I do not mean just sodium chloride, but all bio available mineral salts and electrolytes), they are more sensitive when these salts and electrolytes are incomplete or missing.
For this I recommend Tropic Marin Reef Salt from Germany (which is sold by the pound).
Product Resource: AAP-Tropic Marin Pro Reef Salt from Germany
Further reference: Wikipedia; Brackish Water
A FEW FACTS, USES AND MYTHS ABOUT SALT IN FRESHWATER AQUARIUMS
- Fact, based on much research and my own experience both anecdotal in 1000s of aquariums and with experiments, salt can and does help or even cure some fish maladies such as Columnaris or simple sores.
HOWEVER salt is NOT a cure all and often fails as a salt only Ich treatment, especially with certain fish. Tests also show that use of sodium chloride for Ich often will take longer to affect a cure, often resulting in fish death as this treatment is simply not effective enough for a virulent Ich infestation.
The facts are most uses of sodium chloride salt in freshwater are better for prevention or mild treatment (with the possible exception of a major university study in treatment of Columnaris already noted earlier).
The improper use of salt does not make it a "dated method" any more than Newton's law of gravity. So when aquarium keepers with little real science based experience in fish disease treatment and prevention use this argument, especially since is usually just a small part of many effective treatments, I would suggest simply ignore them as the science and credible experience states otherwise. - Salt is best used therapeutically with most fish, not on an ongoing basis. Long term use can detrimentally affect osmoregulation in most freshwater fish.
As well salt (sodium chloride) best used in the average community aquarium (not brackish fish) these two ways:- Temporary fish baths, often combined with other therapies including antibiotics and Methylene Blue.
- Therapeutically in community aquariums during times of stress where by sodium chloride is only introduced when new fish are added, injury, or sick fish. Then the salt (sodium chloride) is slowly removed again.
As an example, Sodium Chloride is a proven treatment for Columnaris (even in salt sensitive catfish) as per a University of Auburn study.
In the end, keeping sodium chloride in an aquarium all the time will not help with disease resistance in freshwater fish, better is the use of mineral Cations that lower oxidative stress (such as AAP Wonder shells) It is noteworthy that the constant use of sodium chloride can actually increase a fish' susceptibility to disease and parasites by keeping the fish somewhat stressed all the time, which along with oxidative stress, weakens the immune system.
Further Reading:
*Fish Baths, Dips, Swabs
*Columnaris in Fish
*Aquarium Redox & Oxidative Stress in Fish - With NaCl (common sodium chloride salt), NaCl draws water/fluids through the fish often helping osmoregulation or draining tissues that have to much fluid present. Sodium Chloride (as well as Epsom Salts) can help with the generation of fish' essential slime (mucoprotein) coat.
However too much NaCl can also dry tissues too much, especially if over used over extended periods of time or too much salt is used even for a shorter time. Too much sodium chloride or long term use can also inhibit the product of the fish' slime (mucoprotein) coat, so there is a fine line as to the use of NaCl for slime coat generation.
This of course depends upon the fish species and their ability to deal with salt (NaCl). - There are other salts/electrolytes such as Calcium Carbonate that are important for proper osmoregulation, disease prevention (and even treatment) in freshwater aquarium fish.
- Sodium Chloride can be safely used with these other minerals/salts/electrolytes such as the before mentioned Calcium Carbonate as well as Magnesium and other critical minerals. Wonder Shells are an excellent source of these other mineral Cations (electrolytes) and can safely be used with aquarium salt (sodium chloride).
Product Reference:
Unique Wonder Shells by AAP - Sodium Chloride use, even temporary, does not take the place of stronger treatments when needed. Nor does sodium chloride salt take the place of good water maintenance practices, whether it be water changes, use of UV Sterilization, or optimum water parameters that include a balanced Redox.
- Sodium Chloride is useful for Brown Blood disease (nitrite poisoning) in freshwater fish as well as for a stress reliever in fish transport. A minimum chloride concentration of 20 ppm is recommended to prevent nitrite toxicity.
- Sodium Chloride salt can help prevent and even treat many diseases from Ich to Columnaris, however this is an area where many go overboard too much the other direction as sodium chloride is NOT a cure all, especially when it comes to virulent Ich infestations.
However even though salt is not a cure all, salt can certainly improve results when used in combination with other treatments or in fish baths/dips.
As well many Ich infestations and Columnaris infections have been treated successfully with only salt and Salt/Methylene Blue baths.
Further Disease Treatment Information:
Fish Diseases | How to Treat Sick Fish An important read prior to ANY TREATMENT of sick fish
Aquarium Ich, Ichthyophthirius multifilis; Identification, Treatment, Life cycle
Fish Baths; Including the Use of Salt - Sodium Chloride Salt can be safely used in most all freshwater aquarium applications provided proper levels are observed and as noted earlier other “salts” are also employed either with or without sodium chloride.
- Sodium Chloride aids in slime coat generation (often better than over touted slime coat products, especially those that place a slime bandage on the fish). However it should be noted that there are other electrolytes that aid in slime coat generation as well potassium. This said, with most freshwater fish, I still only recommend using salt on a temporary basis.
Please see this article for more about water conditioners:
Aquatic water Conditioners - Sodium Chloride does not dissipate, meaning that generally only water changes will remove salt.
For example if you are using salt (NaCl) in your 20 gallon aquarium and you change 5 gallons of water you need ONLY add salt for the 5 gallons changed, not the 20 gallons of aquarium water otherwise your salt levels will build up with time.
It is noteworthy that very small amounts of Sodium Chloride are used in biological processes within the aquarium/inhabitants, however this amount is not worth adding more salt for.
In the case of some other salts such as Calcium carbonate and potassium, these will get depleted a higher rater by biological processes and will often need to be replaced depending on water change amounts, bio load, tap water (or other replacement water chemistry), tests, etc. - Table Salt is not a deadly poison to fish as some will say (I have read this on Yahoo Answers).
Table salts have anti-caking agents (often with silicates) and sometimes iodine.
Neither of which are deadly poisons.
However use of table salts is best in temporary conditions such as baths, not the main aquarium due buildup of these added ingredients which can increase algae growth or increase iodine to levels (over time) that may be harmful. - Water Softener salt is generally JUST sodium chloride (always check the bag for added ingredients), so its use when all you need is simply sodium chloride salts is perfectly fine (& economical).
The myth that the use of water softener salt will make your tank water “soft” (low GH) is simply incorrect when used normally.
A water softener simply uses sodium chloride (salt) as part of a process whereby calcium and magnesium ions in the water are replaced with sodium ions.
To do the ion replacement, the water in the water softener is run through a bed of small plastic beads or through zeolite. (which is why water from softener should never be used in an aquarium).
Think of it this way; if you eat sugar you do not get sweeter, your body simply converts the sugar into energy or converts then stores the sugars (in other forms).
Further Reference:
Why to NOT use Water Softener Water in Aquariums
Some Suggested Salt Sources for when salt is used in a freshwater aquarium:
- Plain Salt (Sodium Chloride) such as water softener salt:
This is best used when one finds the need for the addition of salt to Amazon River or SE Asia biotope community aquariums, where no additional minerals are needed (other than supplied via water changes and products such as Wonder Shells or Replenish). - SeaChem Cichlid Salt:
This is best used as a standard every day additive in African Cichlid, livebearer, or similar aquariums. This has all essential minerals and Cations, but includes Sodium Chloride as a very nominal non-therapeutic percentage/level. - Marine Salts:
Products such as Instant Ocean or Tropic Marin Premium Reef Salt can be used in place of plain salt (sodium chloride) for before mentioned African Cichlid, livebearer and similar aquariums when salt is needed at a therapeutic level and additional minerals would also be quite helpful. This includes use in Fish Baths and dips.
In fact in short term fish baths, a quality marine salt will improve your results and is recommended for fish bath use for all freshwater fish!
Premium Marine/Reef Salt Resource: Tropic Marin Pro Reef Salt from Germany Is Of The Highest Quality
SALT CONVERSIONS
Often salt amounts are given as mg/L, however milligrams is a measure of weight while most of us use dry measurements such as teaspoons or tablespoons which are measurements of volume. So the average weight of salt must be found before converting.
To convert 1000 mg of salt into a given volume (in this case, teaspoons), you would need to find the average weight per this volume, which in this example is .22 teaspoons per 1000 mg (approximately).
This means that if your treatment required 2000 mg/L you would need .44 teaspoons of salt per liter of water. Since 1 gallon= 3.785 liters, you would need 1.66 teaspoons per gallon of water.
Many salt treatments call for as much as 3000 mg/L which means you would need approximately 2-1/2 teaspoons per gallon.
A few more conversions:
- 1000 grams = 1 gram
- 1 gram = .0353 ounce
- 1 ounce = 28.35 grams
- 1 fluid ounce = 6 teaspoons
- 3 teaspoons = 1 tablespoon
SUMMARY; Further Information about Salt use, both Positive and Negative
I will sum this up by saying that those who say you should not use sodium chloride salt in your freshwater aquarium (including with Catfish, Tetras, etc.) and those who say you should always use salt (NaCl) are only HALF RIGHT!
I would certainly agree that there was (and still is) a segment of the aquarium keeping hobby that thinks sodium chloride is the cure all for everything all the while ignoring important other “salts” such as Calcium Chloride.
However the current “No Salt” fad propagated by many poorly researched articles/blogs/ocial Media posts that sadly come up in Google Searches also ignores certain facts not only about sodium chloride but about the other important electrolytes as well.
While many fish such as Tetras do well without any added salt but for occasional treatment levels or baths, other fish such as Goldfish have been proven to to be more disease resistant with small amounts salt present along with even more important positive mineral ions of elements such as calcium.
My own years of experience and tests along with research and many of my professional aquarium keeping colleagues bears this out.
When salt is used the use of iodized table salt that is often found in kitchen cupboard is not the best choice, however in a pinch it is not a terrible choice as is often described (another aquatic myth).
If the small amount of salt that is needed in a freshwater aquarium is used the iodine (which is also a necessary nutrient for fish in small quantities as in humans) is not likely to cause any problems (due to the trace amount of iodine present after dilution in water).
Common table salt also has anti-caking agents such as sodium alumino silicate which is main reason to avoid table salt as this ingredient may cause increases algae growth (other anti-caking ingredients include potassium ferrocyanide and calcium carbonate).
Table salt is usually fine for short duration dips or baths, I would simply not recommend using table salt long term in an aquarium due to build up over time of anti-caking agents & iodine (assuming iodized salt).
I prefer to use either plain rock salt (water softener salt), marine salt (of which the additional major and trace elements are actually beneficial to many freshwater fish), or products such as SeaChem Cichlid Salt which (similar to marine salt in concept) contains added minerals/electrolytes of which when used in the small amount of salt one should use sodium chloride are actually beneficial for the vast majority of freshwater fish.
Product Resource: SeaChem Cichlid Salt; All Freshwater Fish Safe
However, I do want to clarify that I recommend the use of plain salt (such as regular aquarium salt, water softener salt, etc.) for use in salt and medicated baths over marine or Cichlid salt as the later two add other elements that although essential in the general environment, they could dramatically alter the difference in the bath water and display tank water so to cause osmotic stress/shock.
Another point as to the use SeaChem Cichlid Salt or Marine salt in freshwater aquariums is that these salts BOTH add carbonates/bicarbonates (for KH) and in general many freshwater aquariums need not or even should not be used with buffers that also increase carbonates/bicarbonates.
The use of these two salts is fine with products such as Wonder Shells as these mineral blocks tend to dissolve as minerals are depleted and combined use is highly unlikely to increase GH/mineral levels to anything remotely dangerous for ANY freshwater fish.
In African Cichlid tanks I have often combined buffers AND Cichlid Salt, however I recommend monitoring KH and pH so as to find the “sweet spot” as to the correct amount of each to add so as to keep correct parameters (again the use of Aquarium Mineral blocks such as Wonders Shells is not a concern and I in fact strongly recommend this!)
Other Related Resources/References:
*www.int-res.com/articles/dao/21/d021p171.pdf
*Freshwater Aquarium Basics
*Fish Osmoregulation
*Aquarium Redox
Other Recommended Reference & Product Sites/Videos

The most effective medication BAR NONE for the treatment of Columnaris in an aquarium when used as part of the four step program of Columnaris treatment.
A more synergistic combination than purchasing Kanamycin & Nitrofurazone separately.
AAP Spectrogram; Synergistic Kanamycin/Nitrofurazone

Fish Diseases | How to Treat Sick Fish

YouTube; How to: 4 Steps Columnaris Treatment Fish Bacterial Infection
This video goes over the basics of the full four step plan of properly treating Columnaris in aquarium fish and is a compliment to a FULL reading of this article.

Aquarium and Pond Information, Help, Advice Articles

Aquarium Lighting Facts & Information

POND CARE INFORMATION; Complete Steps
*Planaria & Detritus Worms in Aquarium

Freshwater Aquarium Care; Basics to Advanced

Fish Nutrition
Complete information from fish food building blocks to sources and much more

"Clay Neighbor's AAP Premium All Natural" Premium Optimized Custom Fish Food Crumbles
Made in the USA & sold out of Oregon;
Superior to ALL other fish Foods in quality of optimization of ingredients!
TMC V2 RO Filter systems; the very best you can buy with TDS meter:
 Reverse Osmosis Aquarium Water Filters; with TDS Meter
Reverse Osmosis Aquarium Water Filters; with TDS Meter
A good compliment to RO water or for any freshwater aquarium to add ESSENTIAL Mineral Ions:
*Wonder Shells, Mineral Block

AquaRay Ultra Premium Aquarium LED Lights
Highest in PUR, The ONLY LED with an IP67 rating or higher for water proofing along with a full 5 year warranty to back them up!
Why purchase brands without this rating such as the Finnex, Current, or Fluval only to be essentially placing an electronic light emitting device over your humid aquarium with little or no guarantee? In the long term, you WILL PAY MORE!
* Aquarium Power Head Pumps
Aquarium Power Head Pumps
Superior to Hagen or Marineland, yet more economical.
ADVERTISEMENT
Labels: aquarium salt, aquarium salt use, electrolytes, fish osmoregulation, freshwater aquarium salt, salt, salt fish treatment, sodium chloride
Tap water in Aquarium/Pond; Chlorine/Chloramines, TDS, Vitamin C
By Carl Strohmeyer
Updated 1/22/19
Index (click to "Jump To")
- Chlorine & Chlorimines
- Vitamin C for Chlorine & Chlorimines
- Inorganic Molecules; Nitrites, Nitrates, Copper, Phosphates, and Fluoride
- TDS (Total Dissolved Solids)
- Sodium
- Summary & References
There are standards for tap water quality, but that does not mean that these levels are safe for fish (or humans for that matter).
CHLORINE AND CHLORAMINES:
To start, most city tap water has chlorine (Chlorine (Cl2), Sodium Hypochlorite NaClO), which is an oxidizer (A chemical substance that gains electrons in a redox chemical reaction), but this can kill fish by burning their gills and poisoning their blood.
More about: Aquarium Redox Balance
Chlorine is not very stable and is easily removed with the many commercial "De-chlorinators" available, most using Sodium Thiosulfate.
Agitation of the tap water in a bucket or other container with an air stone connected to an air pump will generally remove the amount of chlorine generally added to tap water in a matter of hours if not at least a day. However, if chloramines are used, agitation will not work for removal.
Some municipalities use chloramines because they are more stable than chlorine (this is especially common in areas where water must be transported over longer distances due to non-availability of local water sources, such as in the Southwest USA or areas of drought).
Chloramines (NH2Cl) are a chemical compound of chlorine and ammonia and cannot be boiled out. It can't even be allowed to sit for a few days to remove the Chloramines before adding this water to an aquarium.
Chloramine is formed through the reaction of dissolved chlorine gas and ammonia in tap water. Chloramines can also be composed of two other formulas: dichloramine (NHCl2) and trichloramine (NCl3).
Chloramine passes through the gills of fish and enters the blood stream. There, it reacts with Hemoglobin, forming Methemoglobin.
In studies of some fish exposed to 1 ppm-Cl of monochloramine, then about 30% of the hemoglobin is converted into methemoglobin; the fish suffered from anoxia (low oxygen in their tissues) because they have lost some of their hemoglobin, which is responsible for carrying oxygen in the blood.
In my experience, fish exposed to chloramine suffer immediate and often severe reactions from darting, to gasping, to immediate shock and death!
This is NOT the general reaction of exposure to chlorine, as fish generally do not show symptom of exposure to chlorine in normal tap water doses unless exposure is prolonged, and most de-chlorinators remove chlorine instantly/within seconds.
See a simple experiment in this article: “Aquarium (& Pond) Water Conditioners” (about three paragraphs down).
ADVERTISEMENT
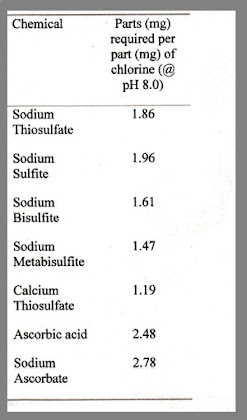 If your tap water has Cloramines, you will need to remove them chemically before adding the water to your aquarium. Standard de-chlorinators such as "Start Right" Water Conditioner will remove the chlorine, but leave the ammonia (NH3) for either your bio filtration or Zeolite (freshwater only) to remove. These basic de-chlorinating products are simple Reducers (sodium thiosulfate) and are quite safe, even overdosed contrary to some opinions floating around.
If your tap water has Cloramines, you will need to remove them chemically before adding the water to your aquarium. Standard de-chlorinators such as "Start Right" Water Conditioner will remove the chlorine, but leave the ammonia (NH3) for either your bio filtration or Zeolite (freshwater only) to remove. These basic de-chlorinating products are simple Reducers (sodium thiosulfate) and are quite safe, even overdosed contrary to some opinions floating around.
The vastly preferred products for use in conditioning water treated with Chloramines such as Amquel (or better Amquel Plus) or SeaChem Prime will remove the chlorine and neutralize the ammonia (and more).
Prime is made from Hydrosulfite salts which are basically non toxic reducing agents made up of bisulfites and hydrosulfites, aqueous solution, buffered at pH 8. As mentioned earlier, reducing agents are basically non toxic at reasonable doses to fish and aquatic animals.
A product resource for: SeaChem Prime
The chart to the right shows some common chlorine/chloramines reducing agents.
You will note that metabisulfites and bisulfites are efficient reducers, however it should be noted that some studies have shown these to lower dissolved oxygen levels. I have never had a problem with this due to the fact I always employ good circulation when ammonia, chloramines, or chlorine are a problem (actually good circulation should always be employed).
Also note, Vitamin C (ascorbic acid/sodium ascorbate) is also an reasonable reducer (albeit not as strong as others at doses that do not affect pH dramatically), which also goes along with many of my points for a Reducing Redox.
In fact, Vitamin C in either Ascorbic Acid or Sodium Ascorbate can effectively lower chlorine or break the chloramine bond. Generally, approximately 2.5 to 2.8 parts of Vitamin C is required to neutralize 1 part chlorine
Be aware that Vitamin C, in particular in the form of ascorbic acid, can dramatically lower pH in normal doses to be effective.
Vitamin C may be a good choice for 25% water changes where its lower reduction abilities at safer pH doses might even be desired.
HOWEVER, in larger water changes or especially with chloramines, the undesirable drop in pH at doses required may make it a poor or even dangerous choice. Ascorbic Acid at doses that may be required will immediately reduce pH by 1.5 on the logarithmic pH scale (which can shock or even kill fish when this change happens suddenly, which it will without adequate alkalinity via bicarbonate buffers). Sodium Ascorbate will change pH at doses required will immediately reduce pH by .5 (reference 1).
Nor is Vitamin C as a good a choice for long term Redox balance and reduction since it is a short term reducer.
For much more information about water conditioners that will remove Chlorine and/or Chloramines, please read this article:
Aquarium (& Pond) Water Conditioners.
Further Aquarium Redox Information:
Aquarium Redox
Other Methods for Chloramine Removal Include:
- Reverse Osmosis or Deionization Resins: reverse osmosis systems (where carbon is usually part of the pre-filtration prior to the RO membrane), the ammonia is partially removed by the reverse osmosis system. The extent of removal by the RO membrane depends on pH. At pH 7.5 or lower, reverse osmosis will remove ammonia from 1.4 ppm-Cl monochloramine to less than 0.1 ppm ammonia.
DI resin can then remove any residual ammonia.
This method is EXCELLENT for preparation of freshwater prior to adding a marine salt mix or for topping off aquariums prior to evaporation.
HOWEVER, it's not a good method for 100% use of water in freshwater aquariums as ALL important minerals are stripped from the water that freshwater fish need for osmoregulation (in marine tanks, the saltwater mixes provide these essential elements).
PLEASE read these articles fully before using this method for freshwater aquariums:
*AQUARIUM CHEMISTRY; Use of RO water in Freshwater Aquariums
* Use of RO, DI, Softwater in Aquariums
*PROPER OSMOTIC FUNCTION- ELECTROLYTES; Osmoregulation in Fish - Carbon & Zeolite: as with thiosulfate (used in may water conditioners such as Start Right, Novaqua, Tap Water Conditioner, etc.), carbon removes the chlorine, however ammonia is not bound significantly by activated carbon.
Consequently, treatment of water with activated carbon will need to be followed up by some method of eliminating the ammonia such as the use of Zeolite. Zeolite is NOT for use in saltwater, however it can be an effective albeit SLOW method of removing residual ammonia from the previous Chloramines molecules.
A Product Resource for: Ammo-Carb, Ammo-Chips, Zeolite Products - Even the use of a Category A or B True UV Sterilizer will slowly lower chlorine in an aquarium as per my tests, albeit not as fast as traditional water conditioners or Vitamin C.
Reference: Aquarium/Pond UV Sterilizer Use
INORGANIC CHEMICALS; Nitrites, Nitrates, Copper, Phosphates, and Fluoride:
Ammonia: As of the time of this article, there are no limits as per the amount of ammonia (either NH3 or NH4) allowed in tap water.
Reference: Ammonia in Drinking Water
Nitrites are allowed up to 1 ppm, yet at this level there can be some damage to fish gills. Methylene Blue (for nitrite and ammonia poisoning) can be used for treatment of nitrite poisoning, but it is best to avoid this. A good bio filter will generally remove trace amounts of this from tap water, as will products such as Prime.
Nitrates are allowed up to 10 ppm, yet at levels above 10- 30 (depending on studies) in human studies infants under 6 months can become ill and suffer symptoms such as Blue Baby Syndrome.
More about:
Aquarium Nitrates
Also See these links for more about Nitrates:
*www.thirteen.org/edonline/studentstake/water/schoolwater/nitrogen/nitrate.htm
Now this level has shown no ill effect in any fish studies I have seen, but levels above 20 ppm can harm some marine cephalopods.
It makes since in many marine aquariums too use RO water to mix up your salt mix or top off for evaporation so as to not add to "difficult to remove nitrates" in you marine aquarium.
Other "allowed chemicals" of note are Copper- 1.3 ppm, Phosphates (no standards) and Fluoride- 4.0 ppm.
Copper at these levels is not generally a problem with fish or aquatic invertebrates, but if you are already treating with copper sulfate or if this is allowed to accumulate in a reef tank this is something an aquarist should be aware of.
Copper levels above 5 ppm can start to become dangerous for some delicate invertebrates such as Acropora corals and levels above 25 ppm can be dangerous to fish. It also should be noted for copper, that in hot water in particular, copper can be also added to tap water via home copper plumbing.
As for Fluoride; I have not found conclusive studies on the harm of Fluoride to fish or other aquatic creatures, in fact trace amounts are necessary for coral growth in marine aquariums. So despite some over stated worries about Fluoride in tap water used in aquariums, this in one I would not consider.
As for Phosphates; many municipalities use phosphates to reduce the levels of lead that have been found in drinking water.
Phosphates create a protective film on the inside of the pipe, slowing the electrochemical processes that lead to corrosion.
Unfortunately for aquarists this can lead to extra algae growth, especially of Blue Green Algae (Cyanobacteria). This can be a real problem in both freshwater and saltwater aquariums without easy solutions.
I have used many phosphate sponges with mixed results, but I can say with certainty is that carbon will not remove phosphate, in fact some carbon may even add to your phosphate levels. Protein Skimmers in marine aquariums can remove some phosphates, but I have not recorded that much difference.
Water changes using RO water and then adding minor elements and electrolytes back in is another solution. In freshwater aquariums, Wonder Shells can help add these elements as well, but in saltwater the marine mixes have all the elements you need.
A resource for:
*“Wonder Shells – calcium and electrolyte replenisher”
*Rena Phos-Zorb
*NPX Bioplastics Nitrate & Phosphate Reducing Polymer Media
TDS (Total Dissolved Solids)
Total Dissolved Solids basically is any minerals, salts, metals, cations (positively charged mineral ions) or anions (negatively charged mineral ions) dissolved in water.
This includes anything present in water other than the pure water (H20) molecule and suspended solids. (Suspended solids are any particles/substances that are neither dissolved nor settled in the water, such as detritus).
The TDS is equal to the sum total of cations and anions ions in the water. Generally the measurement of TDS is given in Parts per Million (ppm) being the weight-to-weight ratio of any ion to water.
There of coarse is a relationship to GH (General Hardness) and Redox, so too low or too high a TDS can be detrimental, depending upon the fish kept. With this in mind, the use of RO (Reverse Osmosis Systems which lower TDS considerably should take into consideration the re-mineralization of the water (again depending upon the fish kept).
Also Redox is affected when one uses RO (or DI) water, as the Redox of RO Water is generally too high (since RO water is more acid oxygen is left behind), as a highly oxidizing environment may be OK for short term, long term health considerations for fish (or even humans as per Redox Research) is not good.
For Further in depth information about Redox:
Aquarium Redox
RO Machine Links:
Reverse Osmosis Water Filter
Aquarium Minerals for RO Water
The diagram below shows the relationship of TDS and Tap Water (from www.tdsmeter.com/)

Please Click on the Diagram Above to Enlarge
SODIUM:
The Environmental Protection Agency suggests 20 mg or less of sodium per liter as the amount of sodium to strive for in drinking water, so anything over this amount is going to tend toward driving out certain minerals, albeit in small amounts, that are essential for fish, such as Calcium & Magnesium. As freshwater sodium level increases beyond this number, it will become increasing difficult to maintain a healthy/balanced GH/KH.
A sodium level that is over 270 mg/liter in freshwater is considered quite high and at this level maintaining the correct essential mineral ions will be very difficult in your aquarium.
It is for this reason a sodium softened water system should NEVER be used for ANY aquarium.
See: Softened Water; Home/Office Water Softeners Use
SUMMARY:
Before you go and rush out and use nothing but bottled water, please note that most bottled water is NOT suitable for fish when used 100% (it can be mixed or reconstituted).
Drinking Water in particular is generally RO water with some minerals added for “taste” (Spring Water is generally fine if it is true spring water). Not that there is anything wrong with RO or DI water, it is just they are devoid of VERY important electrolytes and trace elements needed for proper fish respiration and osmotic function, without which you may be worse off in terms of fish health than with slightly polluted tap water.
So please use the information in this article to improve your water quality and make wise choices as to your water sources.
Please read this article about Aquarium electrolytes and more:
AQUARIUM CHEMISTRY; CALCIUM, KH, AND MAGNESIUM IN AQUARIUMS
Further Resources
- For a more in depth article about Aquarium Test Kits, please follow this link:
AQUARIUM TEST KITS; what they are used for and their importance - "Chlorine and the Reef Aquarium"
- Using Vitamin C To Neutralize Chlorine in Water Systems
Carl Strohmeyer copyright- 1/22/19
Other Recommended Reference & Product Sites

Aquarium or Pond UV Sterilization

Aquarium & Pond Information and Resources

Fish Diseases | How to Treat Sick Fish
*Fish Nutrition for Aquariums
TMC V2 RO Filter systems; the very best you can buy with TDS meter (far superior to 4 stage RO/DI systems sold via Bulk Reef Supply, Amazon, or eBay that use the inferior cellulose triacetate membrane made by Dow):
 Reverse Osmosis Aquarium Water Filters; with TDS Meter
Reverse Osmosis Aquarium Water Filters; with TDS Meter
*Aquarium Ich; Ichthyophthirius Multifilis and Cryptocaryon Irritans
*Complete Pond Care
*The Aquarium Nitrogen Cycle; COMPLETE
*Melafix Dangers? Bettas, Gouramis

UV Replacement Lamps/Bulbs
For TRUE High Output, Hot Cathode, Low Pressure UVC Germicidal Bulbs, not the low output medium pressure bulbs commonly sold at Amazon or eBay
ADVERTISEMENT
Labels: aquarium, Chloramines TDS, Chlorine, City Water, electrolytes, pond, sodium, Tap Water Conditioner, Total Dissolved Solids







