Our Facebook Page to Follow: Aquarium/Pond Answers Facebook
This is a unique resource for answers, help, & advice to aquarium and pond questions not found elsewhere; With regular posts & article updates.
In our research; we use aquaculture, horticulture, medical, & university research to compile many of our articles.Our Recommended Lighting for highest efficiency professional planted/reef aquariums: "AquaRay Lighting"
Do Fish Drink? Osmoregulation in Fish
PROPER OSMOTIC FUNCTION- ELECTROLYTES; REVERSE OSMOSIS & SOFT WATER USE IN AQUARIUM, HOW DO FISH DRINK
INDEX of this article:
this includes Freshwater, Saltwater
& Anadromous/ Catadromous/ Amphidromous Fish
Use of RO, DI, Softwater in Aquariums
By Carl Strohmeyer-PAMR 40+ years experience
Updated 10/14/18
QUESTION: "How do fish drink water?"
ANSWER:
Freshwater fish absorb most of the water they need through their skin via osmosis by producing dilute urine and actively transport essential mineral ions from the surrounding water to compensate for mineral ions lost via the urine and diffusion from the gills (osmosis is the net movement of water through a selective permeable membrane from a region of low solute potential to a region of high solute potential due to their hyperosmotic environment), NOT through their gills. The gills are for respiration.
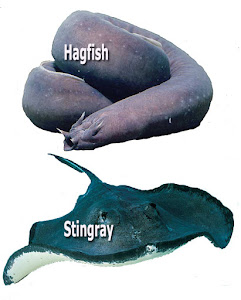 Most saltwater fish (Hagfish, Sharks, Rays differ in osmoregulation) actually drink the water the live in, as the salt in the water is constantly pulling H2O from their bodies in a reverse respiration as their tissues are hypotonic compared to surrounding water and they must ingest large volumes of water and actively excrete mineral ions (hypo-osmotic environment).
Most saltwater fish (Hagfish, Sharks, Rays differ in osmoregulation) actually drink the water the live in, as the salt in the water is constantly pulling H2O from their bodies in a reverse respiration as their tissues are hypotonic compared to surrounding water and they must ingest large volumes of water and actively excrete mineral ions (hypo-osmotic environment).
Marine Bony fish (not Rays, Sharks Hagfish) maintain their osmotic concentration at about one quarter to one third that of sea water.
Normally in salt/sea water, bony marine fish have a tendency to lose water from their gills due to osmosis as well through their urine. Marine fish have to drink a lot of water to make up for the loss, however, as the water contains a lot of salt (35% or approximately 1.025 specific gravity) they must remove the excess salt from their system.
The sodium and chloride ions are secreted by the gills and magnesium and sulphates are excreted in urine.
An important side note is this is an active process and requires much energy which is why lowering salt levels (NOT other mineral ions) can be helpful in aiding a sick bony marine fish!
It is noteworthy that Hagfish, Sharks, Rays have similar concentrations of salts to that of marine bony fish, however, they also have very high concentrations of organic compounds which gives their internal fluids the same osmotic concentration as sea water so these fish due not generally benefit from lower salt levels when sick.
These different abilities explain why some fish such as catfish are sensitive to salt in the water, but this is also why some fresh water fish are helped by salt to generate a mucous slime coat on their skin which is necessary for disease prevention.
For proper osmoregulation electrolytes such as positive ions of calcium, magnesium and other elements are important as well.
This is important and not realized by many aquarists (especially in freshwater), however not having these electrolytes present in the water whether by depletion or by the use of water marketed as "drinking water", distilled water, or RO water that has NOT been re-mineralized can cause problems with the fish’ ability to move fluids in and out of their bodies and in the long term resist disease.
This difference also explains why when one adds commercial vitamin preparations to a freshwater aquarium, the dilution renders it 99% useless since freshwater fish absorb much more slowly than bony saltwater fish "drink". Such vitamin preparations should only be used in a food soak or similar (unless one is into wasting money by feeding the entire water column).
Another note, because most freshwater fish cannot drink their surrounding water (Salmon and others are exceptions), when you place these freshwater fish in saltwater, they actually dehydrate.
ADVERTISEMENT
Osmosis in fish;
Their cells must always be bathed in a solution having the same osmotic strength as their cytoplasm. This is one of the reasons why fish and other animals have kidneys. The exact amount of water and salt removed from their blood by the fish kidneys. The process of regulating the amounts of water and mineral salts in the blood is called osmoregulation. Fish which live in the sea (remember the sea is full of salt and other elements), but fish which live in freshwater have the opposite problem; they must get rid of excess water as fast as it gets into their bodies by osmosis. Osmosis is an important topic in biology because it provides the primary means by which water is transported into and out of cells.
Osmosis is also important in the treatment of many aquarium diseases in both freshwater and saltwater. A general breakdown in osmoregulation due to disease, poor water quality (especially the lack of essential mineral electrolytes such as Calcium, magnesium, and Sodium) is often responsible for the bloated condition that results from excess water accumulation in tissues. This lack of proper osmoregulation can not only result in bloating, it can cause issues with disease resistance, curvature of the spine, and the ability of the fish to stabilize itself in the water.
It is common for Fish in this condition often rapidly succumb due to loss of homeostasis (the constant internal environment), essential to carrying out metabolism and other life activities. This tends to be more common among FW fish in my experience, in part due to the lack of understanding of the role that many essential minerals play in essential life processes of fish.
Generally salts (trace elements), not just sodium chloride can affect osmosis. Magnesium can also play a major role too. Calcium can affect and just as importantly BE affected by proper osmotic function.
Sulfates have been shown effective in improving nutrient absorption and toxin elimination.
Magnesium plays a role in the activity of more than 325 enzymes and aids in the proper assimilation of Calcium.
Often many aquarists understand how salt (sodium chloride) affects osmoregulation and the popular question of “Do fish drink?” however this is a dangerous over simplification as although sodium chloride (as often represented as sodium and chloride) are important, the lack of OTHER ESSENTIAL ELEMENTS including Calcium in fish can lose/leak substantial quantities of other minerals/salts into the water.
Here is an over simplification I read recently that is not necessarily wrong, but in misleading in that it implies salt is the only essential mineral:
“They (freshwater fish) absorb water through their skin and have effective ways of excreting excess liquid to maintain the salt they need”.
The implication is that the fish basically just needs to maintain salt and/or this or other minerals somehow take care of themselves.
The FACTS are that without Calcium (as tested via GH), the fish CANNOT properly osmoregulate.
For much more information about the importance of Calcium and other electrolytes, please read this article (in particular the section about Calcium):
CALCIUM, KH, AND MAGNESIUM IN AQUARIUMS; Why Calcium and Electrolytes are Important.
FRESHWATER FISH
In freshwater, a higher electrolyte level (particularly of sodium chloride, calcium and magnesium) will help pull fluids through the body which also stimulates the natural mucous coat on fish so as to resist parasites, bacteria, and fungus.
Also by pulling fluids through the body this can help with bloat, swim bladder problems, intestinal problems, and even dropsy (which I have had few problems with in clean tanks with good electrolyte/ trace element levels).
This process results in the loss of many electrolytes. Some of these trace elements can be replaced by ions contained in food but by far the most common method is through the movement of a substance against an osmotic gradient through the use of energy.
This usually involves the exchange of one substance for another. In the case of freshwater fish, Na+ (sodium) ions are taken from the water and ammonia ions are taken from the fish and they are exchanged.
This effectively rids the fish of ammonia. Chloride ions are exchanged for carbonate ions which help in maintaining the pH of the body fluids.
This is one more important reason for adequate Calcium, carbonate (KH), & electrolyte levels
Opportunistic diseases such as Columnaris, Saprolegnia (often known as fish fungus), and Aeromonas (often the cause of Septicemia are more easily prevented when osmoregulation is functioning properly in fish via adequate mineral levels.
Information on:
Columnaris, Saprolegnia
Aeromonas
However it should also be noted that before you go an dump a lot of sodium chloride (salt) in your freshwater aquarium, even for fish such as African Cichlids where this is a common practice, that overuse of salt can have negative side effects such as loss of other essential elements/minerals and general osmoregulation (& is occasionally noted as a contributing factor for the condition of Malawi Bloat).
Put another way, you want to achieve an Osmotic balance.
I prefer to use salt in fish such as African Cichlids sparingly and then bring it “up” during times of stress or suspected disease, only to bring it to low levels such as just a 1 teaspoon per 5 gallons reserving higher salt concentrations for 30 minute “salt baths” of 1-2 teaspoons per gallon when necessary for treatment/therapy.
What I (and many good research papers) find is that maintaining healthy levels of Calcium, magnesium, sodium, and Carbonates is far more important for long term osmoregulation and fish health, whether it be African Cichlids or Discus. The LACK of these other elements are often a common factor in cases of Malawi Bloat or even HITH in Discus.
See also: Hole in the Head (HITH) Disease in Fish
For much more about the correct use of salt (NaCl) in freshwater aquariums, please see this article:
“Aquarium Salt (Sodium chloride) in Freshwater Aquariums; both pro and con
SALTWATER FISH
A saltwater (marine) fish in water with a slightly higher salt level will devote more molecular energy to osmoregulation. Following this logic, if a fish is suffering from stress lowering the salt level will help the fish to recover.
Lowering the salt level (salinity) will reduce osmotic pressure within the fish allowing it to allocate more of its molecular energy to its immune system, this will help stop the stressed fish from becoming sick and can be used to treat fish that are sick.
As well, in marine fish sometimes lowering salinity to a certain point will have a reverse osmotic effect and rupture the cell wall of many parasites such as Oodinium and Cryptocaryon (this is best achieved in a 3-5 minute freshwater bath adjusted for pH).
This method of lowering the specific gravity (salt content) in saltwater to fight disease should not be taken too far.
I have heard of persons being told to keep their marine aquariums at a specific gravity of 1.012 to prevent or fight disease, however this is TOO LOW. At his specific gravity (salinity), the marine fish will not have proper osmotic function (remember, marine fish drink the water around them and at this salt level they will not get the fluids and minerals being pulled properly through their bodies which can result in water retention and MUCH worse).
The general specific gravity in marine aquariums should be around 1.019 to 1.024 for fish and around 1.022 to 1.025 for reef.
Be careful when lowering salinity as marine fish can generally only handle about 2 degrees of salt change per day (example; 1.025 to 1.023).
To treat parasite infections (such as Oodinium and Cryptocaryon), you can TEMPORARILY and slowly lower the specific gravity to as low as 1.010.
Be careful in lowering salinity with corals and anemones present as they cannot tolerate the lower salinity levels fish can.
ANADROMOUS, AMPHIDROMOUS, & CATADROMOUS FISH
 These are fish that can migrate between fresh and saltwater.
These are fish that can migrate between fresh and saltwater.
- Anadromous fish live in the sea mostly, and then breed in fresh water such as the Pacific Salmon
- Catadromous fish live in fresh water and then breed in the sea such as freshwater eels of genus Anguilla
- Amphidromous fish move between fresh and salt water during some part of life cycle, although not for reasons of breeding such as Bull sharks in the Zambezi river of Africa and living in Lake Nicaragua of Central America.
Using Salmon as an example of how bony fish (other than sharks, rays, true eels) osmoregulate in these dual conditions;
Salmon spend most of their life in the open ocean, where they reach sexual maturity, but lay their eggs gravel beds at the upper reaches of freshwater streams.
When the eggs hatch, the young salmon spend several months migrating downstream to the ocean where they remain for some 3-5 years. When mature, the adult salmon return to mouth of stream where they hatched migrating upstream to its headwaters, spawn, and die.
There are osmoregulation/ physiological challenges presented by habitats as different as freshwater streams and the open ocean for which the salmon must adapt to so as to complete this cycle. The salmon uses adaptations, both behavioral and physiological, that allow it to thrive in both fresh and salt water habitats.
To offset the dehydrating effects of salt water, the salmon drinks copious amounts of saltwater.
So as an ocean-dwelling salmon drinks, it takes in a lot of NaCl, which exacerbates the salt-loading problem. However in fresh water the salmon doesn't drink at all.
Kidney function also differs between the two habitats.
In fresh water, the salmon's kidneys produce large volumes of dilute urine (to cope with all of the water that's diffusing into the salmon's body fluids), while in the ocean environment, the kidneys' urine production rates drop dramatically and the urine is as concentrated as the kidneys can make it.
The result of this is that the salmon is using relatively little water to get rid of all of the excess ions it can.
Finally, the other adaptation salmon use to deal with the NaCl fluxes driven by the gradients between the salmon and its surroundings is in their gills.
In their gill epithelial cells, salmon have a special enzyme that hydrolyzes ATP (Adenosine triphosphate).
ATP is the universal unit of energy used in all living cells and a salmon uses the released energy to actively transport both Na+ and Cl- against their concentration gradients.
In the ocean, these Na+-Cl- adenosine triphosphatase molecules 'pump' Na+ and Cl- out of the salmon's blood into the salt water flowing over the gills, thereby causing NaCl to be lost to the water and offsetting the continuous influx of NaCl.
In fresh water, these same Na+-Cl- adenosine triphosphatase molecules 'pump' Na+ and Cl- out of the water flowing over the gills and into the salmon's blood, thereby offsetting the continuous diffusion driven loss of NaCl that the salmon is subject to in fresh water habitats with their extremely low NaCl concentrations.
When a young salmon on its seaward journey first reaches the saline water at the mouth of its home stream, it remains there for a period of several days to weeks, gradually moving into saltier water as it acclimates.
During this time, it begins drinking the water it's swimming in, its kidneys start producing a concentrated, low-volume urine, and the NaCl pumps in its gills literally reverse the direction that they move NaCl (so that they're now pumping NaCl out of the blood and into the surrounding water.
Likewise, when an adult salmon is ready to spawn and reaches the mouth of its home stream, it once again remains in the brackish water zone of the stream's mouth until it is able to reverse the changes it made as a juvenile invading the ocean for the first time.
Further reference:
www.unm.edu/~toolson/salmon_osmoregulation.html
Necessary Minerals
Here are a few necessary TRACE elements/minerals (electrolytes) and their function.
- Calcium (Actually needed in more than trace amounts): Calcium helps to transport ions (electrically charged particles) across the membrane, is essential for muscle contraction, calcium assists in maintaining all cells and connective tissues in the body, and much more.
With many fish, long term lack of calcium can result in the inability of the fish to properly balance and swim correctly due to poor muscle contraction ability or swim bladder issues. These symptoms can mimic diseases such as Whirling Disease when in fact there is no actual disease. Unfortunately, sometimes when a fish gets to this point, correcting Calcium and other essential mineral levels is too little, too late.
Please read for more about Calcium:
Use of RO, DI Softwater in Aquariums
Aquarium Chemistry; Calcium
For a professionally proven method to maintain calcium and essential mineral Cations in ALL freshewater fish:
AAP Wonder Shells; Fresh, NOT Clearance Stock as sold elsewhere by discounters/Amazon - Sodium (Actually needed in more than trace amounts which is why water from home water softeners should NOT be used): Regulates extra-cellular electrolyte, essential for the transport of nutrients across the cell membranes.
- Potassium: Regulates intracellular osmotic pressure, cell membrane potential, and salt excretion.
- Phosphorus: Energy metabolism.
- Molybdenum: Important for proper skeletal growth (very important in reef aquariums for hard coral growth).
- Manganese: Aids enzymes involved in metabolism, growth and maintenance of bone and cartilage.
- Iron: Oxygen transport in blood and muscle tissue.
- Magnesium: As stated previously, magnesium plays a role in the activity of more than 325 enzymes and aids in the proper assimilation of Calcium.
- Sulfates: Also as stated above, improve nutrient absorption and toxin elimination.
- Chromium: Important for proper utilization of sugars.
- Cobalt: Necessary for Folic Acid synthesis.
- Copper (very trace amounts): Co-enzyme for energy metabolism, aids in the protection of the myelin sheath around the nerves, important for iron absorption and utilization.
For a related post that deals with trace elements:
“Plaster in Paris in Aquariums and Ponds”
It is important to have a proper Redox Potential which describes the ability for the loss of an electron by a molecule, atom or ion to the gain of an electron by another molecule, atom or ion.
Without this reducing Redox Potential many minerals cannot be absorbed and properly assimilated. So it is very important to keep a “positively charged” aquarium or a Balanced Redox Potential via proper dissolved oxygen levels, calcium and other electrolytes, proper cleaning procedures and water changes (UV Sterilization can help too).
For more information about the Redox Potential, please see this article:
*The Redox Potential in Aquariums (& Ponds) and how it relates to proper aquatic health
For further reading on this subject, here are a few articles I recommend:
This other Aquarium Answers article is an excellent compliment:
*Tap water for my Aquarium or Pond? From Chlorine and Chloramines to Phosphates & TDS
This now deleted article is a very interesting (although somewhat dated), especially as it pertains to the benefits of lower salinity for Marine teleost/teleostei fish (ray-finned fishes):
*http://www2.hawaii.edu/~delbeek/delb11.html (now a dead link)
This point from this article is noteworthy:
"Since marine fish must constantly expel various solutes, such as sodium and chloride ions, against an osmotic gradient, a great deal of energy is required. Therefore, anything that you can do to lower the osmotic gradient will benefit the fish in terms of energy expenditure. The simplest way of doing this is to lower the salinity of the water as much as possible, particularly for a fish in distress."
*Can you say Anadromous, Catadromous, Amphidromous, Oceanodromous, or Potamodromous?
For more aquarium information, please visit this site:
* “AQUARIUM AND POND INFORMATION”
*Sodium Ions: http://www.elmhurst.edu/~chm/vchembook/142Aposion.html
Carl Strohmeyer- Copyright 2016
Other Recommended Reference & Product Sites

Aquarium Information and Resources (Pond too)
*UV Sterilization, Sterilizer Use
The above article is the most in depth and constantly researched/updated article to be found ANYWHERE on the Internet!

Fish Diseases | How to Treat Sick Fish

TMC Total Dissolved Solids Monitor
An excellent stand alone product for testing your TDS from Tap, well, or even aquarium water.
A must have for any advanced aquarium keeper.
*Ich; Lifecycle, Identification, Treatment, Prevention

AquaRay Ultra Premium Aquarium LED Lights
Highest in PUR, The ONLY LED with an IP67 rating or higher for water proofing along with a full 5 year warranty to back them up!
Why purchase brands without this rating such as the Finnex, Current, or Fluval only to be essentially placing an electronic light emitting device over your humid aquarium with little or no guarantee? In the long term, you WILL PAY MORE!
*Pond Care
*Aquarium Forum; Everything Aquatic & Aquarium Forum Board
 FISH AS PETS
FISH AS PETS
Fish as Pets contains articles and commentary of Interest to the Aquarium Hobby
The Best in Value UV Sterilizers:
*Aquarium & Pond UV-C Sterilizers/Clarifiers
 COMPACT UV STERILIZERS, Terminator 7 watt to 36 watt
COMPACT UV STERILIZERS, Terminator 7 watt to 36 watt
*TMC PREMIUM High Dwell Time Pond/Aquarium UV Sterilizers
ADVERTISEMENT
Labels: Amphidromous, Anadromous, Catadromous, Do fish drink, osmoregulation, osmotic function in fish, trace minerals for fish







