Use of Activated Carbon in Freshwater, Marine Aquariums and Ponds
Index:
- How Activated Carbon Works
- Common Carbon Uses
- Lignite Activated Carbon
- Chemical Properties
- Contaminant Properties
- Water Temperature and pH
- Exposure Time
- Possible Concerns with Carbon Use
- List of compounds carbon can or cannot absorb
By Carl Strohmeyer-PAMR 40+ years experience
Updated 1/23/20
 Carbon is primarily an adsorbent which is a very popular chemical filter media that is often misunderstood as to use in established aquariums and ponds as well.
Carbon is primarily an adsorbent which is a very popular chemical filter media that is often misunderstood as to use in established aquariums and ponds as well.
A healthy established aquarium (fresh or salt) with regular water changes generally needs little carbon (although more carbon is generally needed in marine reef aquariums and less in low pH freshwater aquariums).
Carbon will NOT remove or absorb ammonia, nitrites, or nitrates. Carbon is very useful in removing medications after treatment or even between doses.
Please read the entire article for a good understanding of what activated carbon can or cannot do for your aquarium/pond; including the table of what carbon can and cannot remove as well as the referenced resources
How Activated Carbon Works
Activated carbon has an extremely large surface area per unit weight, which makes AC an extremely efficient absorptive AND adsorptive material.
The activation of carbon and its manufacture create many pores within the particles, and it is the vast areas of the walls within these pores that account for most of the total surface area of the carbon.
In water, activated carbon has a preference for large organic molecules and for substances which are non-polar in nature.
The forces of attraction between the carbon and the absorbed molecules are greater the closer the molecules are in size to the pores. The best absorption takes place when the pores are just large enough to admit the molecules.
Activated carbon, when contacted with water containing organic material, will remove these compounds selectively by a combination of adsorption of the less polar molecules, absorption (filtration) of the larger particles, and partial deposition of colloidal material on the exterior surface of the activated carbon.
As well as absorption, activated carbon uses a process called Adsorption, in fact adsorption is the primary method of molecule removal by carbon, not absorption.
When a material adsorbs something, it means it attaches it by chemical attraction.
The extent of removal of soluble organics by absorption depends on the diffusion of the particle to the external surface of the carbon and diffusion within the porous adsorbent. For colloidal particles, internal diffusion is relatively unimportant because of particle size.
Organic substances that pass through the column consist of hydrophilic organic molecules (substances that are attracted to, and dissolve well within, water) and hydrophobic molecules (repulsed by water).
If the molecule is “polar” (having both a hydrophobic and hydrophilic attributes) which many organic molecules are, the hydrophobic side will be attracted (attached) to the activated carbon.
Adsorption is partially the result of forces of attraction at the surface of a particle that cause soluble organic materials to adhere to the activated carbon. The limited water solubility of many organic substances will affect AC adsorption of these molecules.
Put more simply (I hope): Polar, hydrophobic and hydrophilic interactions are important interactions necessary to understand how activated carbon adsorb the certain molecules.
A Methylene Blue dye molecule is hydrophobic and has a large affinity to the hydrophobic carbon rings of the activated carbon.
The dye prefers to interact with the carbon rather than water.
Where as non chelated metals (such as copper ions) are positively charged (hydrophilic), and the carbon is neutral and hydrophobic. Therefore, the positively charged metal ions prefer to interact with the water, which is hydrophilic. “Like dissolves Like”.
This applies to most metals from the periodic table, including calcium, magnesium, etc., and for this reason most essential minerals are not removed by activated carbon unless chelated but they can loose their positive charge due to oxidative processes of the Redox Potential, which is another reason to replenish these ESSENTIAL positive mineral ions, especially if carbon is used.
This positive/negative ionization is why DOC (organics) will also negatively affect the Aquarium/Pond Redox Balance
Please see this article for further information:
The Aquarium Redox Potential
ADVERTISEMENT
Common Uses
As already noted healthy aquariums often do not need much activated carbon, however this is a rather ambiguous generalization.
An aquarium keeper needs to consider DOC (dissolved organic compounds/carbon), pH, desired compounds that can or cannot be removed, bio load of the aquarium or pond, and desired environment.
As an example, an Amazon River aquarium will generally need less or no carbon while a heavily fed reef tank will need more.
One reason for little or no carbon in an Amazon River aquarium is these are generally low pH environments that are high oxidizing with little or no Redox Reduction. As noted earlier, positively charged metal ions once they give up their positive charge are easily removed and in a low pH, highly oxidizing aquarium this can be done rapidly. The use of Carbon, especially in larger quantities will only speed this process.
I have made many tests and observations over the years in freshwater and marine aquariums of different environments, as well as ponds as to how much carbon is best to use (if any).
I would use tests of pH/KH and nitrates to help determine need. Although carbon cannot remove nitrates, it can remove most DOC that will eventually end up as nitrates.
So a falling pH/KH and climbing Nitrate level would generally be an indicator more activated carbon or some carbon is needed.
Redox tests if available can sometimes be helpful since activated carbon can act as a Redox Mediator.
See this article for further reference:
Redox as it Pertains to Aquariums; Including Methylene Blue Test
Product Source: Methylene Blue
Simply gauging activated carbon use by tank water color, if the tank is yellowing this can be an indicator of the need for more carbon. However this is not a 100% accurate indicator, nor can activated carbon remove all the causes of yellow water.
The use of carbon if only for a day or even hours after or between medication treatments is another important aspect of carbon use.
In Reef aquariums, the use of carbon (even though less effective in higher pH water) is important in my experience/opinion as part of a regimen that where carbon is but one piece of the aquarium maintenance puzzle that often includes Protein Skimmers (which also remove many similar DOC, but not as quickly after production), water changes, micron filtration, de-nitrification with deep sand beds or products such as Matrix, and use of chemical absorbents such as Purigen.
Product Sources:
*TMC V2 Premium Marine Aquarium Protein Skimmer
*SeaChem Matrix
*SeaChem Purigen premium synthetic absorbent

Carbon can also be used in mixed products such as Ammo Carb, but zeolites are only for freshwater use.
The use of zeolite/carbon mixes is especially useful in areas where municipal tap water contains chloramines (instead of the usual chlorine).
I have also achieved good results with carbon/zeolite blends in ponds which are generally exposed to even more contaminants than aquarium.
Further Information: Municipal Tap Water, Chloramines
Product Source: Ammo-Carb from AAP
A good starting point for activated carbon use (please note that this is a generalization), is one to three teaspoons per ten gallons of water.
One teaspoon of activated carbon is equal to approximately 6 grams in weight measurement. This amount will vary greatly depending upon many other factors that the aquarium or pond keeper can determine.
As well sometimes an aquarium keeper may choose to only use this amount of carbon as part of a clean up procedure (in particular freshwater aquariums) and then discontinue use after a day or two.
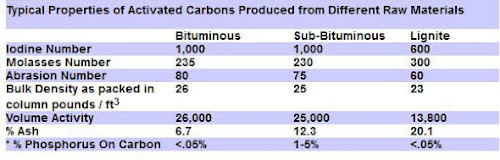
Lignite Activated Carbons
 The best Premium Activated Carbon is produced from a soft, brownish-black coal (Lignite) in which the alteration of vegetable matter which has proceeded further than in peat but not as far as in bituminous coal.
The best Premium Activated Carbon is produced from a soft, brownish-black coal (Lignite) in which the alteration of vegetable matter which has proceeded further than in peat but not as far as in bituminous coal.
Lignite based carbon is the best choice for use in aquarium & ponds to remove organic molecules, pesticides and for color removal, due to its large pore size.
Product Source: High Grade Lignite Pelletized Carbon from AAP
The medium to large pore size is important, because the organics in a aquarium or pond environment will clog and render ineffective, the smaller-pored, coconut shell carbons.
6 grams (.21 ounces) of Lignite Activated Carbon has the surface area of a football field, so a little goes a long way in aquarium use in particular.
The other common coal based carbon are the Bituminous coal activated carbons. These are harder with more varied pore sizes.
The graph below compares some of the coal based carbon properties:
Here are a few aspects that impact the effectiveness of activated carbon
Chemical Properties;
The carbon surface may actually interact chemically with organic molecules. As well electrical forces between the activated carbon surface and some contaminants may result in adsorption or ion exchange.
Adsorption, then, is also affected by the chemical nature of the adsorbing surface. The chemical properties of the adsorbing surface are determined to a large extent by the activation process.
Activated Carbon formed from different activation processes will have chemical properties that make them more or less attractive to various contaminants.
Contaminant Properties:
Large dissolved organic compounds/carbon (DOC) are most effectively adsorbed by activated carbon. A general rule of thumb is that similar materials tend to associate. DOC molecules and activated carbon are similar materials; therefore there is a stronger tendency for most organic chemicals to associate with the activated carbon in the filter rather than staying dissolved in a dissimilar material like water.
Generally, the least soluble organic molecules (such as large complex amino acids or fatty acids) are most strongly adsorbed. Often the smaller organic molecules (such as sugars) are held the tightest, because they fit into the smaller pores.
It is also noteworthy that although larger complex organic molecules (often nitrogen based) are more readily absorbed, these molecules are also not held as tightly and re-leased under certain conditions which is why carbon should not be relied on for the sole form of organic contaminant removal.
Other methods such as Purigen, de-nitrifying filters, water changes, Protein Skimmers (marine aquariums), micron filters, UV Sterilizers, etc. should be employed as part of the mix.
Concentration of organic contaminants can affect the adsorption process. A given activated carbon may be more effective than another type of activated carbon material at low contaminant concentrations, but may be less effective than the other carbon material at high concentrations.
Water Temperature and pH;
Adsorption usually increases as pH and temperature decrease. Chemical reactions and forms of chemicals are closely related to pH and temperature. When pH and temperature are lowered many organic chemicals are in a more absorbable form (this is noteworthy for marine/saltwater use and why Protein Skimmers are also important as these devices will remove DOC as well, although not immediately as carbon can)
Exposure Time;
The process of adsorption is also influenced by the length of time that the carbon is in contact with the contaminant in the water. Increasing contact time allows greater amounts of contaminant to be removed from the water. Contact is improved by increasing the amount of activated carbon in the filter and reducing the flow rate of water through the filter.
There is controversy in what essential minerals carbon will absorb or what activated carbon will or will not absorb in general. I will state based on my own experience and scientific evidence that carbon has many uses in aquariums/ponds but is also over used or incorrectly recommended. Although I use little carbon in my established healthy aquariums and ponds, I disagree with those that state it should not or rarely be used (based on some false assumptions of what carbon removes or adds to water).
On the flip side I also disagree with those that make carbon the answer for water quality issues such as nitrates for which carbon does not remove.
Activated Carbon is very useful for removing most medications after or between treatments (this is where I strongly recommend its use), although even here, carbon does not remove most copper formulations effectively.
Possible Concerns with Carbon Use
*Activated carbon can foster the growth of bacteria by concentrating other organics (such as DOC) on its surface.
Although I have not performed controlled tests to confirm this, I have made many observations over the years that increased use of carbon has coincided with increased incidence of bacterial infections such as Aeromonas, especially in lower pH Amazon River & SE Asia water aquarium environments since Redox is also generally less favorable and the removal of tannins by carbon from products such as Indian Almond Leaf Extracts allows these bacteria to thrive.
The fact that activated carbon removes oxygen, further increases the risk of an opportunistic Aeromonas Bacterial outbreak since these bacterium are anaerobic.
Further References:
*Aeromonas, Septicemia, Vibrio Infections in Aquarium Fish
*Aquarium Chemistry; Amazon River Water Environments
Product Source: Atison's Spa, Indian Almond Leaf Extracts, lowers Aeromonas
Use in planted freshwater aquariums:
This is an area of some controversy of which some information is based on facts, some information is not, with some reasonable questions in between.
The main controversy I will address for now as to carbon use in planted freshwater aquariums is the removal of trace minerals. I read some experiments at “the Krib”, as well I have made observations and tests (as well as research) over the years myself.
The main testable point is that most metals such as Iron (which is important for plants) are NOT absorbed carbon with an important and noteworthy exception; and that is the use of chelation.
EDTA (which is an organic molecule) is used to chelate many metals such as iron to make it more readily available for fertilizers or other uses, and since activated carbon is especially effective in removing organic carbon based molecules, these chelated metals are then removed.
Any aquatic plant fertilizers that contain chelated metals will be bound to the carbon pores, and as result their concentration into the water column will get lower with the use of activated carbon.
If the carbon is left in the aquarium for a period of time, the chelated compounds in aquariums slowly decay and release their metals.
However not all trace elements are chelated, for instance SeaChem Flourish uses water soluble non-chelated iron, as well mineral blocks such as Wonder Shells are non chelated and any possible absorbed trace minerals are rapidly replaced by the Wonder Shell (which although mineral depletion by activated carbon is low, the use of Wonder Shells in aquariums/ponds utilizing activated carbon insures adequate minerals)
Product Sources:
*SeaChem Flourish Plant Fertilizer
*Wonder Shells; Unique Version ONLY available at AAP
The use of Activated Carbon with Marine Protein Skimmers:
Although conclusive tests are forth coming, there is evidence that the use of activated carbon can limit the amount of foam refraction generated by a marine protein skimmer. This is likely due to the adsorption of Foaming Agents (MBAS) by activated carbon.
This presents a problem for many reef keepers since both carbon and protein skimmers are useful aspects of a complete marine filtration system.
My suggestion is to limit carbon use in cleaning filters run during certain times of the day (or week), especially after heavy feeding.
The re-use of carbon after removal during disease treatment:
Carbon can trap many pathogens, so soaking in saltwater (specific gravity of 1.025) while out of the aquarium can prevent re-infection once added back into the aquarium.
HOWEVER, this is far from 100% sure, especially with viruses, and some bacteria and certain fungi (generally the saltwater soak is very effective to kill parasites).
Soaking in bleach/chlorine or any other Redox oxidizer is not a viable alternative since (as per the table below), carbon is very good at removing these products. What will happen is these will cancel each other out; either the amount of bleach or similar used will exhaust the carbons capability or the carbon will remove all the oxidizer this not allowing any disinfection.
*A final concern with activated carbon is the possible release of contaminants after they have been initially adsorbed. This action is known as desorption or dumping. This could occur if other ambient water quality characteristics change.
Although at the time of writing this article, I have not discovered the exact mechanism for causing this, but I do know that a tank that is not stable in its general chemistry, whether pH or Redox is a candidate for this possible problem of carbon use.
Here is a list of compounds carbon can or cannot absorb.
Please note that some compounds may be desirable to remove depending upon your tank requirements while the same compound may not be desirable in another aquarium environment.
A good example would be tannins (which carbon removes reasonably well); in a soft water environment an aquarist would likely want to restrict the use of Activated Carbon, while someone keeping “dirty” goldfish may find the use of carbon on a regular basis (in higher quantities) a necessity.
| WHAT CARBON CAN ABSORB: | ||
|---|---|---|
| Excellent Absorption: | Fair/Good Absorption | WHAT CARBON CANNOT ABSORB (or absorption is poor) |
| *Amyl Acetate *Amyl Alcohol *Benzene *Bleach *Butyl Alcohol *Butyl Acetate *Calcium Hypochlorite *ORGANIC carbon *Chloral *Chloroform *Chlorine *Chlorobenzene *Chlorophenol *Cresol *Defoliants *Diesel Fuel *Dissolved Organic Compounds *Dyes (such as Methylene Blue or Acriflavin) *Ethyl Acetate *Ethyl Acrylate *Foaming Agents (MBAS) *Gasoline *Glycols *Herbicides *Hydrogen Peroxide *Hypochlorous Acid *Insecticides *Iodine *Isopropyl Acetate *Isopropyl Alcohol *Ketones *Methyl Bromide *Methyl Ethyl Ketone *Naptha *Nitrobenzene *Nitroluene *Odors (general) *Oil - dissolved *Organic Esters *Oxalic Acid *Oxygen *PCB's *Pesticides *Phenol *Sodium Hypochlorite *Toluidine *Trichlorethylene *Turpentine *Xylene | *Acetaldehde *Acetone *Alcohols *Antifreeze *Chloramine (only the Chlorine, zeolite needed for remaining ammonia) *Calcium Hypochlorite *Chlorophyll *Citric Acid *EDTA (an organic chelator of metals such as iron) *Ethyl Alcohol *Ethyl Amine *Ethyl Chloride *Etyl Ether *Hydrogen Sulfide *Lactic Acid *Mercaptans *Methyl Acetate *Methyl Alcohol *Methyl Chloride *Organic Acids *Organic Salts *Ozone *Potassium Permanganate *Propioc Acid *Propyl Acetate *Propyl Alcohol *Propyl Chloride *Radon *Solvents *Sulphonated Oils *Tannins, such as: Indian Almond Leaf Extract (Atison's Spa) *Tar Emulsion *Tartaric Acid *Xanthophyll *Cyanide *Copper (this in part depends upon type of copper compound and chelated copper is not readily removed by carbon), also availability of oxygen and a lower pH can improve basic copper sulfate adsorption by carbon. Basically carbon should not be relied for total copper removal, especially in Marine aquariums using chelated carbon *Free, low molecular weight hormones. | *Alkalinity *Calcium *Carbon Dioxide *Fluoride, *Formaldehyde *Hardness. *Lime *Magnesium *Manganese *Microbes, *Molybdenum *Nitrates, nitrites, ammonia *Phosphates *Selenium *Sodium, *Lead, Iron and other heavy metals are removed only by adding a chelation process using EDTA, an organic carbon molecule (then these metals can be readily removed) *Protein bound hormones are generally NOT removed by carbon |
SUMMARY;
It is noteworthy that not all carbons are the same, but hopefully the reader will understand what a quality activated carbon can and cannot do.
There are many excellent carbons available on the market, as well as many very poor carbons/charcoals.
My advice is if the carbon you are currently using is not matching the results expected by the above chart, then switch.
As well, do not feel stuck using manufacturer pre-filled carbon bags that are commonly sold for many filters. My experience has been that the majority of these are of poor quality, and both results and cost savings can be achieved by purchasing a separate carbon and refillable bag to use in your filter.
Product Examples:
High Grade Lignite Pelletized Carbon from AAP
Lees Filter Bags
References
*The Krib; Activated Carbon
*Copper & Cyanide Removal by Carbon
Other Recommended Reference & Product Sites

Aquarium Lighting, Information
The largest data base of aquarium lighting information available on the Web, which with Googles latest updates, good information is nearly impossible to find.

Fish Treatments, How They Work, Which to Use and Not to Use

NilocG Aquatics; Planted Tank Liquid Ferts from AAP
NilocG Aquatics PROFESSIONAL GRADE planted aquarium liquid fertilizer products.
Products designed for persons who want to have a more advanced planted aquarium without the hassles need for a degree in science to do so. Meant for use in "The Estimative Index of Dosing, or No Need for Test Kits" method of planted aquarium aquascaping.

Eheim Everyday Automatic Feeder
The EHEIM Everyday Fish Feeder is a compact fish feeder with actively aerated feeding chamber.
This unit includes a clamp for fixing to open top aquariums or terrariums. Easy to understand programming, double dosage of food is programmable
TMC V2 RO Filter systems; the very best you can buy with TDS meter:
 Reverse Osmosis Aquarium Water Filters; with TDS Meter
Reverse Osmosis Aquarium Water Filters; with TDS Meter
A good compliment to RO water or for any freshwater aquarium to add ESSENTIAL Mineral Ions:
*Wonder Shells, Mineral Block
Aquarium Forum; Everything Aquatic
An excellent place to go for information, help or simply to share your love of the aquarium and pond hobby and help others. A superior place for information over such places as Yahoo Answers

UV Replacement Lamps/Bulbs
For TRUE High Output, Hot Cathode, Low Pressure UVC Germicidal Bulbs, not the low output medium pressure bulbs commonly sold at Amazon or eBay
Planaria & Detritus Worms in Aquarium
Melafix Dangers; The Known Facts
Celestial Pearl Danio, Galaxy Rasboras
AAP/SeaChem Tidal High Capacity
Aquarium HOB Power Filter (By Sicce)
Premium "Hang On-the Back" Filtration Systems; Including skimmer feature & high capacity filter basket

Spirulina 20 Fish Food Flake
The best balanced fish flake food diet for Tetras and other fish for disease prevention
ADVERTISEMENT